Solve the problem.The Van der Waals equation provides an approximate model for the behavior of real gases. The equation is P(V, T) = 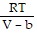 -
- 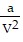 , where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
, where P is pressure, V is volume, T is Kelvin temperature, and a,b , and R are constants. Find the partial derivative of the function with respect to each variable.
A. PV = - 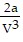 +
+ 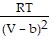 ; PT =
; PT = 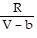
B. PV = 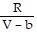 ; PT =
; PT = 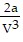 -
- 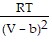
C. PV = 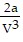 -
- 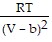 ; PT =
; PT = 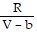
D. PV = 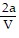 -
- 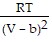 ; PT =
; PT = 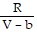
Answer: C
Mathematics
You might also like to view...
Solve the problem.What is the minimum product of two numbers whose difference is 90?
A. -22.5 B. -2025 C. -45 D. -4050
Mathematics
Decide whether or not the set is closed under multiplication.{1, 2, 3, 4, . . .}
A. Closed B. Not closed
Mathematics
Multiply. Write the answer in simplest form.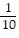 ?
? 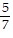
A. 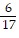
B. 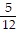
C. 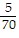
D. 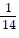
Mathematics
Find the vertical asymptote(s), if any, of the graph of the rational function. f(x) = 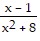
A. x = 1, x = -1 B. no vertical asymptote C. x = -8 D. x = 8
Mathematics