Given the data table below for a binary mixture at 298.15 K in vapor-liquid equilibrium. What can you conclude about the system?
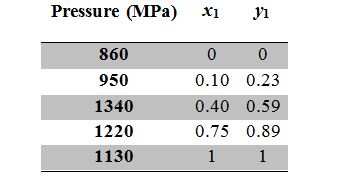
A. The system will have an azeotrope.
B. The system shows positive deviations from Raoult’s Law.
C. The liquid phase is well modeled by the ideal solution.
D. The excess volume for this system will be positive across all compositions.
E. None of the above
A. Incorrect. This seems very unlikely, since the liquid-phase composition continually lags the vapor-phase composition.
B. Correct. The system pressure is sometimes above both pure-component vapor pressures.
C. Incorrect. The system pressure is sometimes above both pure-component vapor pressures, indicating positive deviations from Raoult’s Law.
D. Incorrect. You have no way of determining this from the data given.
E. Incorrect. One statement is correct.
You might also like to view...
Most scientists agree that the greatest danger we have from our food supply is that of food spoilage and the ingestion of bacteria
Indicate whether the statement is true or false
Zeros must be added to the left of the MSB to produce even groups of 4 bits when converting from binary to hexadecimal
Indicate whether the statement is true or false
The length of a standard belt is measured _____
A) on the outside surface B) on either the inside or outside surface C) along the pitch line D) on the inside surface
____ can reduce heat buildup in warm weather, lowering cooling bills.
A. Small appliances B. Large appliances C. Use of stoves instead of a variety of small appliances D. Use of gas instead of electric appliances