A 2-in-diameter spherical ball whose surface is maintained at a temperature of 170°F is suspended in the middle of a room at 70°F. If the convection heat transfer coefficient is 15?Btu/h•ft2•°F and the emissivity of the surface is 0.8, determine the total rate of heat transfer from the ball.
What will be an ideal response?
A spherical ball whose surface is maintained at a temperature of 170°F is suspended in the middle of a room at 70°F. The total rate of heat transfer from the ball is to be determined.
Assumptions 1 Steady operating conditions exist since the ball surface and the surrounding air and surfaces remain at constant temperatures. 2 The thermal properties of the ball and the convection heat transfer coefficient are constant and uniform.
Properties The emissivity of the ball surface is given to be ? = 0.8.
Analysis The heat transfer surface area is
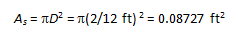
Under steady conditions, the rates of convection and radiation heat transfer are
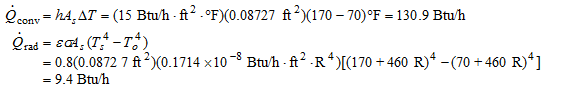
Therefore,
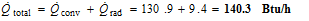
Discussion Note that heat loss by convection is several times that of heat loss by radiation. The radiation heat loss can further be reduced by coating the ball with a low-emissivity material.
You might also like to view...
The throat capacity of a drill press is _____.
a. the largest drill the chuck can hold b. the distance from the table to the chuck c. the distance the drill can be raised and lowered d. the distance from its rear post to the center of the bit
Root ____ absorb the water and nutrients taken in by the root system
A) tips B) hairs C) dermas D) cambiums
You’ve just landed your first job at the US Patent Office in the Mechanical Systems Division. On your first day, your boss gives you a patent to evaluate. After reading the claim, you have determined that the working fluid is air and the system is closed. Also, the air changes state from 300 °C and 2.5 bar to 100 °C and 1 bar, and this change produces 3000 Joules of work per mole of air. Finally, the system rejects heat to a reservoir operating at room temperature (23 C). If one assumes that air is an ideal gas [Cp = (7/2)R] here, does the information provided above give you any basis for rejecting this patent? Please prove, either way. (Hint: Think in terms of a certain “Law” of thermodynamics that could shed light on the feasibility of this process.)
You’ve just landed your first job at the US Patent Office in the Mechanical Systems Division. On your first day, your boss gives you a patent to evaluate. After reading the claim, you have determined that the working fluid is air and the system is closed. Also, the air changes state from 300 °C and 2.5 bar to 100 °C and 1 bar, and this change produces 3000 Joules of work per mole of air. Finally, the system rejects heat to a reservoir operating at room temperature (23 C). If one assumes that air is an ideal gas [Cp = (7/2)R] here, does the information provided above give you any basis for rejecting this patent? Please prove, either way. (Hint: Think in terms of a certain “Law” of thermodynamics that could shed light on the feasibility of this process.)
What is the fundamental difference between the AW analysis that you learned in Chapter 6 and the AW-based replacement analysis that you are learning in this chapter?
What will be an ideal response?