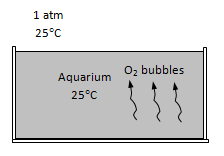
Oxygen gas is forced into an aquarium at 1 atm and 25°C, and the oxygen bubbles are observed to rise to the free surface in 4 s. Determine the penetration depth of oxygen into water from a bubble during this time period.
What will be an ideal response?
An aquarium is oxygenated by forcing oxygen to the bottom of it, and letting the oxygen bubbles rise. The penetration depth of oxygen in the water during the rising time is to be determined.
Assumptions 1 Convection effects in the water are negligible. 2 The pressure and temperature of the oxygen bubbles remain constant.
Properties The mass diffusivity of oxygen in liquid water at 298 K is DAB = 2.4 ×10-9 m2 /s (Table 14-3b).
Analysis The penetration depth can be determined directly from its definition (Eq. 14-38) to be
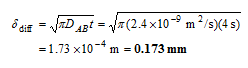
Therefore, oxygen will penetrate the water only a fraction of a milimeter.
You might also like to view...
Which of the following methods are allowed to keep hot water available quickly at a remote plumbing fixture? Select all that apply.
A) Recirculating system using a pump. B) Recirculating system using gravity flow. C) Remote undercounter tank type heater.
Good electrical insulators include:
A) Copper, aluminum, and steel. B) Ceramic, rubber, and plastic. C) Steel, iron, and aluminum. D) Gold, silver, and copper.
Once the problem is well-defined, the design team collaborates. This is a process called ____.
A. evaluation B. brainstorming C. generation D. constraint
 In the accompanying figure, what do the small numbers indicate?
In the accompanying figure, what do the small numbers indicate?
What will be an ideal response?