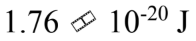Imagine that the energy levels of a certain system are proportional to 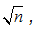 , where n = 1, 2, 3, …. Imagine that for some reason, only transitions such that ?n = 1 are physically possible. In a spectrum chart (like the one shown in figure Q11.2) the emission lines produced by this system
, where n = 1, 2, 3, …. Imagine that for some reason, only transitions such that ?n = 1 are physically possible. In a spectrum chart (like the one shown in figure Q11.2) the emission lines produced by this system
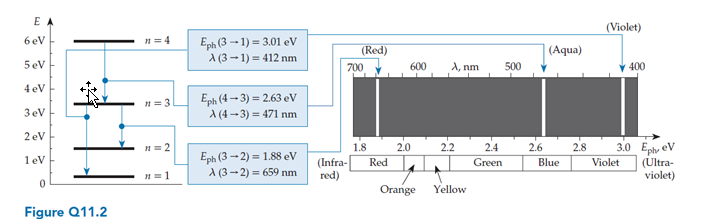
A. Get closer together (in energy) as we go to the right.
B. Are evenly spaced (in energy).
C. Get farther apart (in energy) as we go to the right.
C. Get farther apart (in energy) as we go to the right.
You might also like to view...
Calculate the number of atoms per unit volume in BCC solid sodium (Na) assuming that the lattice parameter for sodium is 0.428 nm.
What will be an ideal response?
Neglecting friction, if a Cadillac and Volkswagen start rolling down a hill together, the heavier Cadillac will get to the bottom
A. at the same time as the Volkswagen. B. after the Volkswagen. C. before the Volkswagen.
A pendulum bob of mass m is set into motion in a circular path in a horizontal plane as shown in the figure. The square of the angular momentum of the bob about the vertical axis through the point P is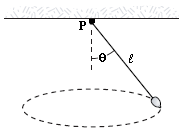
A. m2gl3 sin4?/cos ? B. m2gl3 sin3?/cos ? C. m2gl3 sin2?/cos ? D. m2gl3 sin ?/cos ? E. m2gl3 sin2?
The reaction rate of a chemical reaction doubles when the temperature increases from 10 to 50
to 50 What is the activation energy?
What is the activation energy?
a. 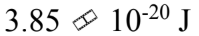
b. 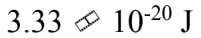
c. 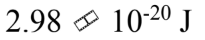
d. 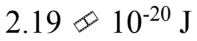
e.