Explain the role of radioisotopes in generating nuclear power. What is spent fuel?
What will be an ideal response?
Radioisotopes emit subatomic particles and high?energy radiation as they decay into lighter isotopes, until they become stable isotopes. Uranium-235 is the radioactive isotope that is used for commercial nuclear power. It is enriched and processed into "fuel rods," which are bombarded with neutrons that split the uranium nucleus. The fission reaction emits more neutrons, smaller atoms which are fragments of the U-235 along with substantial heat and radiation. The released energy is used to create steam. Electricity is generated as the steam spins turbines. After several years in a reactor, uranium decays and cannot generate adequate energy, so it must be disposed of and replaced with new fuel.
You might also like to view...
Which atom is largest?
A. Li B. Na C. H D. Rb E. K
By approximately what year was Australia settled by Aborigines?
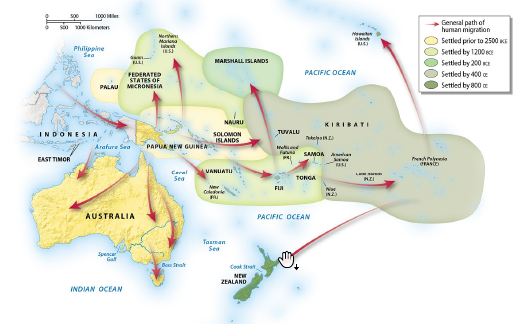
A) by 400 CE
B) by 2500 BCE
C) by 1200 CE
D) by 200 BCE
E) by 1200 BCE
The Virgin Lands Campaign affected this republic the most
A. Moldova B. Latvia C. Kazakhstan D. Tajikistan
Define interface
What will be an ideal response?