A membrane made of 0.15-mm-thick soft rubber separates pure O2 at 1 atm and 25°C from air at 5 atm pressure. Determine the mass flow rate of O2 through the membrane per unit area and the direction of flow.
What will be an ideal response?
Assumptions 1 Steady operating conditions exist. 2 Mass transfer through the membrane is one-dimensional. 3 The permeability of the membrane is constant.
Properties The mass diffusivity of oxygen in rubber at 298 K is DAB = 2.1?10-10 m2/s (Table 14-3b). The solubility of oxygen in rubber at 298 K is 0.00312 kmol / m³.bar (Table 14-7). The molar mass of oxygen is 32 kg / kmol (Table A-1).
Analysis The molar fraction of oxygen in air is 0.21. Therefore, the partial pressure of oxygen in the air is
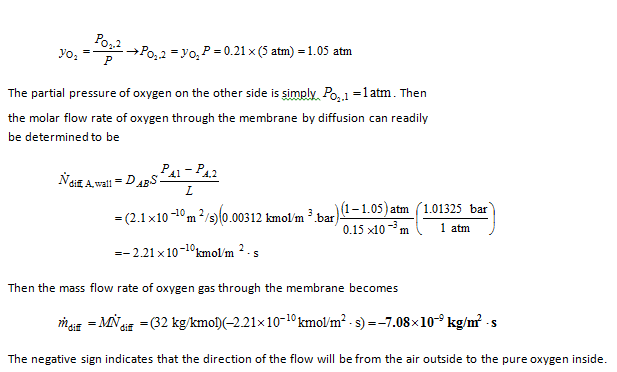
You might also like to view...
A credit card issued by the GECU credit union has an APR of 16% and an APY of 16.64%. (a) What is the compounding period? (b) Use the EFFECT function to find the compounding period.
What will be an ideal response?
In forward contracting, the cattle owner and the processor agree upon the number,
quality, price and future date of delivery of the livestock. A. True B. False
The tool that uses a bubble in a glass vial to check level and plumb is a _____
a. level b. Hi-Lo gauge c. straightedge d. combination square
____________ are typically listed on BOMs.
A. investor names B. part quantities C. lunch breaks D. the boss' names