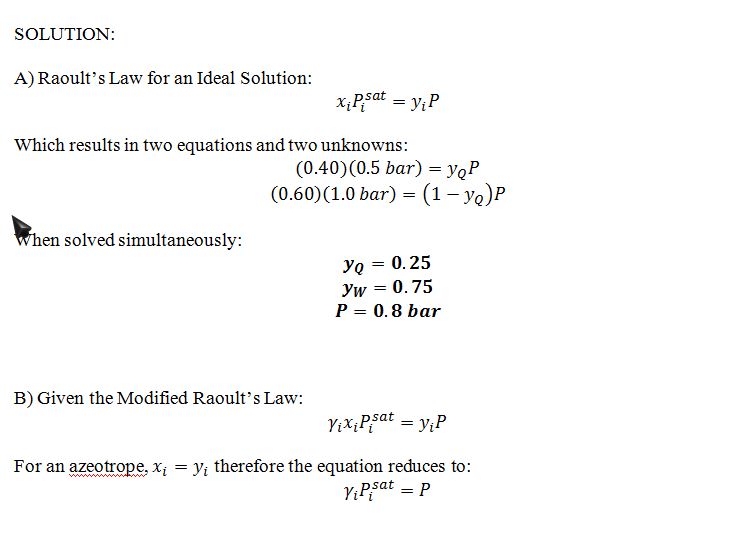
This problem concerns vapor-liquid equilibrium mixtures of compounds Q and W. We know that:
• The vapor pressure of pure compound Q is 0.5 bar at 300 K.
• The vapor pressure of pure compound W is 1.0 bar at 300 K.
A) If a liquid is composed of 40% Q and 60% W and at 300 K, give your best estimate of the bubble pressure and composition of the first bubble of vapor that WOULD be observed IF Q and W formed ideal liquid solutions.
Q and W do not, in reality, form ideal solutions. In reality, for vapor liquid equilibrium at 300 K, there is an azeotrope at P=0.9 bar and T=300 K, in which the liquid and vapor phase both contain 40% Q. Use this fact—as well as the vapor pressure data given above—to answer questions B and C.
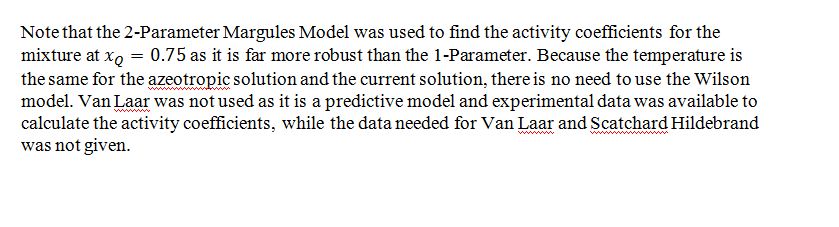
B) Give your best estimate of the activity coefficients of Q and W in the azeotropic mixture at 300 K.
C) Give your best estimate of the bubble pressure and composition of the first bubble of vapor in equilibrium with a liquid that is composed of 75% Q and 25% W at 300 K.


You might also like to view...
What is the minimum formal education requirement for curators?
A) ?bachelor's degree B) ?associate's degree C) ?master's degree D) ?high school diploma or GED
___________________ are used when hydraulic lines must be frequently connected and disconnected.
Fill in the blank(s) with the appropriate word(s).
The cp ____ option creates a symbolic link or name at the destination rather than a physical file.
A. -s B. -y C. -l D. -n
Technician A says a soap solution must only be used in well-ventilated spaces only. Technician B says leaks often occur in areas of limited access where it is difficult to use a halogen leak detector. Who is correct?
A. Technician A only B. Technician B only C. Both A and B D. Neither A nor B