A scheme for finding the internal volume  of a gas cylinder consists of the following steps. The cylinder is filled with a gas to a low pressure
of a gas cylinder consists of the following steps. The cylinder is filled with a gas to a low pressure 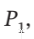 and connected through a small line and valve to an evacuated reference tank of known volume
and connected through a small line and valve to an evacuated reference tank of known volume 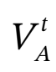 . The valve is opened, and gas flows through the line into the reference tank. After the system returns to its initial temperature, a sensitive pressure transducer provides a value for the pressure change ?P in the cylinder. Determine the cylinder volume
. The valve is opened, and gas flows through the line into the reference tank. After the system returns to its initial temperature, a sensitive pressure transducer provides a value for the pressure change ?P in the cylinder. Determine the cylinder volume 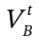
class="w-image" /> from the following data:
What will be an ideal response?
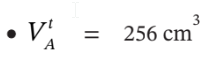
? ?P / P 1 = ?0.0639
Before the valve is opened, when all of the gas is in cylinder B, at pressure 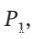 we can write the number of moles of gas (assuming it to be ideal) as
we can write the number of moles of gas (assuming it to be ideal) as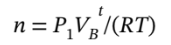 After the gas is allowed to expand into the second tank and return to the initial temperature, we can write the total number of moles in terms of the final pressure
After the gas is allowed to expand into the second tank and return to the initial temperature, we can write the total number of moles in terms of the final pressure 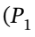 + ?P) and total volume
+ ?P) and total volume 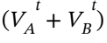 as
as
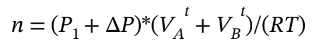
Both the number of moles of gas and the temperature are the same before and after the expansion. So, we can write
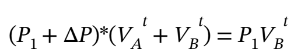
Solving this for the unknown volume 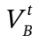 gives
gives
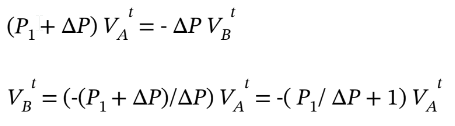
Putting in the numbers, we have
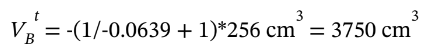
You might also like to view...
____________________ are designed to produce an output voltage that is proportional to the strength of a magnetic field.
Fill in the blank(s) with the appropriate word(s).
Describe the three major agricultural pollutants
What will be an ideal response?
The cat's association with humans dates back to about 3,500 years ago
Indicate whether the statement is true or false
Why is nitrate an issue for human health and the environment?
What will be an ideal response?