What is the power needed to change the speed of a 1600-kg sport utility vehicle from 15.0 m/s to 40.0 m/s in 4.00 seconds?
A) 100 kW
B) 10.0 kW
C) 140 kW
D) 14.0 kW
E) 275 kW
E
You might also like to view...
Other astronomers were skeptical about Percival Lowell's claims of Martian canals because
A) it did not seem possible for his telescope to see the surface through its thick atmosphere B) they discovered that Lowell suffered from poor vision C) they were able to prove that Lowell's telescope was faulty D) when they pointed their own telescopes toward Mars they couldn't see them
What is the power of a lens that has a focal length of -40 cm?
A) -2.5 diopters B) -4.0 diopters C) +4.0 diopters D) +2.5 diopters E) +2.7 diopters
Which of the following telescopes would benefit most from adaptive optics?
A) The Keck I Telescope on Mauna Kea. B) The Hubble Space Telescope. C) The Arecibo Radio Telescope in Puerto Rico. D) The Chandra X-Ray Observatory.
Forty-five kilograms of carbon dioxide is stored in a high-pressure cylinder that is 25 cm in diameter (OD), 1.2 m long and 1.2 cm-thick. The cylinder is fitted with a safety rupture diaphragm designed to fail at 14 MPa (with the specified charge, this pressure will be reached when the temperature increases to 50°C). During a fire, the cylinder is completely exposed to the irradiation from flames at 1097°C (? = 1.0). For the specified conditions, c = 2.5 kJ/(kg K) for CO2. Neglecting the convective heat transfer, determine the length of the time the cylinder may be exposed to this irradiation before the diaphragm will fail if the initial temperature is 21°C and (a) the cylinder is bare oxidized steel(? = 0.79), (b) the cylinder is painted with aluminum paint (? = 0.30).
GIVEN
- CO2 in a high pressure cylinder exposed to flames
- Mass of CO2 (mg) = 45 kg
- Cylinder dimensions
- Outside Diameter (D) = 25 cm = 0.25 m
- Length (L) = 1.2 m
- Thickness (s) = 1.2 cm = 0.012 m
- Rupture diaphragm fails at 14 MPa (Tgf = 50°C = 323 K)
- Temperature of flames (Tf) = 1097°C = 1370 K ( ?f = 1.0)
- Specific heat of CO2 (cv) = 2.5 kJ/(kg K)
- Initial temperature (Tgf) = 21°C = 394 K
FIND
The time for the diaphragm to fail if the cylinder is
(a) bare oxidized steel (?s = 0.79) or
(b) painted with aluminum paint (?2s = 0.30)
ASSUMPTIONS
Convective heat transfer is negligible
Cylinder is 1% carbon steel
Irradiation is constant and uniform over the entire cylinder
Quasi-steady state
Thermal resistance between the gas and the cylinder is negligible (Ts = Tg)
Variation of specific heat of gas and cylinder with temperature is negligible
SKETCH
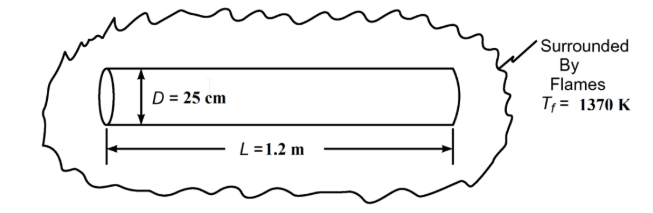
PROPERTIES AND CONSTANTS
the Stephan-Boltzmann constant (?) = 5.67 x 10–8 W/(m2 K4
for 1% carbon steel at 21°C.
Density (ps) = 7801 kg/m3
Specific heat (cs) = 473 J/(kg K)