Determine the equivalent capacitance of the combination shown when C = 12 pF
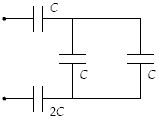
a.
48 pF
b.
12 pF
c.
24 pF
d.
6.0 pF
e.
59 pF
d
You might also like to view...
Ideal Gas Law: A jar holds 2.0 L of ideal nitrogen gas, N2, at STP. The atomic mass of nitrogen is 14.0 g/mol, the ideal gas constant is R = 8.31 J/mol ? K, Avogadro's number is NA = 6.022 × 1023 molecules/mol, and 1.00 atm = 101 kPa.(a) How many moles of nitrogen are in the jar?(b) How many nitrogen molecules are in the jar?(c) What is the mass of the nitrogen in the jar?
What will be an ideal response?
On the scale of the cosmic calendar, in which the history of the universe is compressed into 1 year, when did the dinosaurs become extinct?
A) in late December B) in late November C) in late October D) in late September E) in late August
Two capacitors with capacitances of 2.0 and 3.6 ?F, respectively, are connected in series. The system is connected to a 40-V battery. What charge accumulates on the 2.0-?F capacitor?
a. 220 ?C b. 31 ?C c. 2 100 ?C d. 51 ?C e. 290 ?C
Aquatic life is affected by waste heat
What will be an ideal response?