This problem concerns a compound that has the following physical properties:
Critical Temperature = 1000 K
Critical Pressure = 100 bar
Vapor pressure at 450 K = 4 bar
Molar Volume of saturated liquid at 450 K = 300 cm3/mol
Enthalpy of vaporization at 450 K = 15 kJ/mol
In the VAPOR and GASEOUS states only, it is described by the equation of state:
Z = 1 + BP + CP2
Where B = -0.02 bar-1 and C = 0.0005 bar-2
A) Derive an algebraic (i.e., no differentials or integrals) expression for the fugacity of the compound in the gas/vapor state as a function of temperature, pressure, and known constants.
B) Use the Clausius-Clapeyron equation to calculate the vapor pressure of this compound at 500 K.
C) List the assumptions made in the derivation of the Clausius-Clapeyron equation and comment on how good or bad they are for the example in part B.
D) Give your best estimate of the fugacity of this compound in the LIQUID phase at T=500 K and P=50 bar. You may assume your answer to part B is a correct vapor pressure, even if in part C you expressed reservations about its accuracy.
Apply the equation for fugacity of a gas integrated with respect to P:

Apply Clausius-Clapeyron equation:
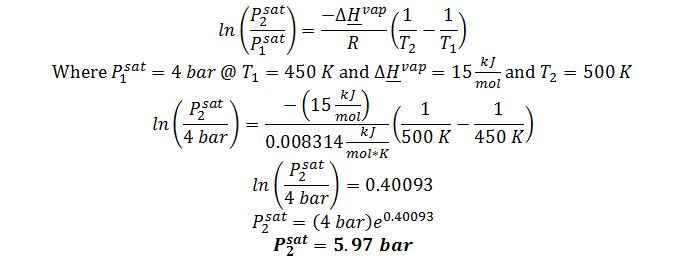
Assumptions for derivation of Clausius-Clapeyron equation:
??H^vap is constant with respect to temperature and pressure
?V^L is negligible in comparison to?V^V
?V^V is well modeled by the ideal gas law
All of these assumptions are marginal in this case. T_1andT_2 are not near the critical temperature of 1000 K but are 50 degrees apart. (When the equation was applied over a 50°C temperature range in Section 8.2.4, the error was approximately 4%.) Assumptions 2 and 3 and marginal in this case as well since the vapor pressures used in the equation are 4-6 bar, conditions at which the ideal gas model would be reasonable for many compounds, but a noticeable departure from ideal gas behavior would be expected for highly polar compounds.
Apply the Poynting correction:
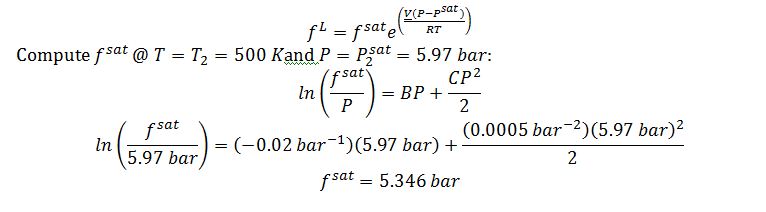
Note that this result suggests the ideal gas model was not valid, since for ideal gases f=P.
Substitute into Poynting correction to solve for f^L @ 50 bar and 500 K:
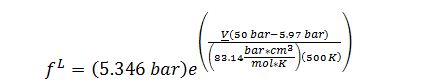
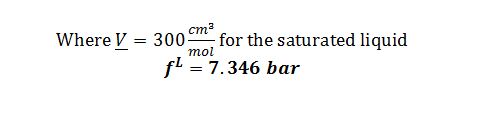
You might also like to view...
What are two main things to look for ahead while driving
Cdl
A hormone called epinephrine stimulates the release, or letdown, process.
Answer the following statement true (T) or false (F)
In regards to a starter motor an one-way clutch is often referred to as:
A. A drive pinion B. Overrunning clutch C. Gear reduction D. A plunger
A duct with a grooved seam measures 7 3/4 inches in diameter and is 33 inches long. It has a seam width of 3/4 inch. Find the width of the stretchout in inches.
Fill in the blank(s) with the appropriate word(s).