Explain the galvanizing process with regard to zinc and its importance to the uses of zinc.
What will be an ideal response?
Zinc's major use is in forming a protective coating over steel to prevent rust. This is referred to as galvanizing. Galvanizing involves placing the steel to be coated into a bath of molten zinc, which bonds to its surface. It is important that the coating be free of imperfections, such as pinholes, that permit moisture to reach the steel and cause it to rust. Both galvanized sheet and strip material are available.
You might also like to view...
If you see something unsafe on the job site, you should _____.
a. try to correct the problem yourself b. report it immediately to your supervisor c. write a report for the Safety Committee d. assume someone else will take care of it
Oxygen may be depleted by hot summer nights
Indicate whether the statement is true or false
The MOD number of a Johnson counter always equals one-half the number of flip-flops in the counter
Indicate whether the statement is true or false
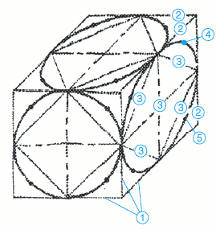 In the accompanying figure, the items identified with a number 1 are the ____.
In the accompanying figure, the items identified with a number 1 are the ____.
A. midpoint of triangles B. diagonals and center lines C. arcs through midpoints D. base lines