Chlorine, Image, is a gas at room temperature, but bromine, Image, is a liquid. Explain
A) Chlorine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
B) Bromine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
C) The smaller chlorine molecules are able to pack together in a tighter physical orientation.
D) The bromine ions are held together by ionic bonds.
Answer: B
You might also like to view...
Which of the following objects would be most likely to produce an emission-line spectrum?
A) a star like our Sun B) a light bulb C) the Earth D) a neon light
If VA ? VB = 50 V, how much energy is stored in the 36-?F capacitor?
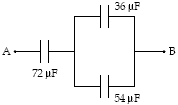
a. 50 mJ
b. 28 mJ
c. 13 mJ
d. 8.9 mJ
e. 17 mJ
An 80-kg man is skating northward and happens to suddenly collide with a 20-kg boy who is ice skating toward the east. Immediately after the collision, the man and boy are seen to be moving together at 2.5 m/s in a direction 60° north of east
How fast was the boy moving just before the collision?
A star begins fusing hydrogen to helium the moment it leaves the main sequence
a. True b. False Indicate whether the statement is true or false