A tank containing 20 kg of water at 20°C is fitted with a stirrer that delivers work to the water at the rate of 0.25 kW. How long does it take for the temperature of the water to rise to 30°C if no heat is lost from the water? For water, 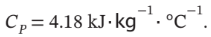
What will be an ideal response?
The total heat capacity of the water is 20 kg? 4.18 kJ/(kg? °C) = 83.6 kJ/°C = 83600 J/°C. So, to increase the temperature by 10°C requires an energy input of 836 kJ. If we are doing work on the water at a rate of 0.25 kW = 0.25 kJ/s, then we will have to do so for 836/0.25 = 3344 s = 55.7 minutes = 0.929 hr.
You might also like to view...
Gaps called _____ are formed where the soil particles touch
a. pores c. chinks b. shales d. holes
Technician A says that the purpose of the intake air temperature (IAT) sensor is to provide the engine computer (PCM) with the temperature of the air entering the engine. Technician B says that the IAT sensor information is used for fuel control (adding or subtracting fuel) and spark timing, depending on the temperature of incoming air. Which technician is correct?
A) Technician A only B) Technician B only C) Both technicians D) Neither technician
When you are passing a vehicle, pedestrian, or bicycle you should assume that:
a. They see your vehicle. b. They may be moving to your traffic lane. c. They know you are to pass.
One of the most commonly used applications of positional tolerancing is in the location of small round holes.
Answer the following statement true (T) or false (F)