A well-stirred vat has been fed for a long time by two streams of liquid, fresh water at 0.2 cubic meters per second and concentrated blue dye at 0.1 cubic meters per second. The vat contains 10 cubic meters of this mixture and the mixture is being drawn from the vat at a rate of 0.3 cubic meters per second to maintain a constant volume. The blue dye is suddenly changed to red dye at the same flow rate. At what time after the switch does the mixture drawn from the vat contain a ratio of red to blue dye of 99:1?
What will be an ideal response?
Solution:
Let the concentration of red dye be denoted by 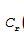 (t) and the concentration of blue dye be denoted by
(t) and the concentration of blue dye be denoted by 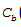 (t) . The concentration of water is constant throughout at 2/3. The rates of change of the dye concentrations are governed by
(t) . The concentration of water is constant throughout at 2/3. The rates of change of the dye concentrations are governed by
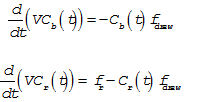
where V is the constant volume 10 cubic meters, 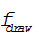 is the flow rate of the draw from the vat and
is the flow rate of the draw from the vat and 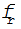 is the flow rate of red dye into the tank. Solving the two differential equations,
is the flow rate of red dye into the tank. Solving the two differential equations,
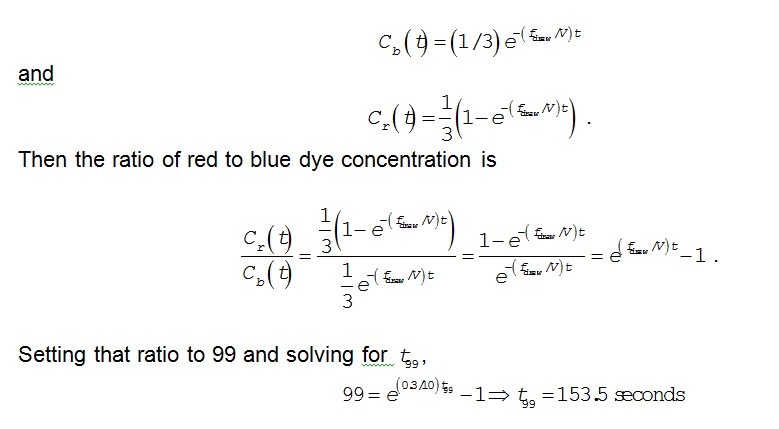
You might also like to view...
What is haylage?
What will be an ideal response?
List the common materials used for vertical X-bracing.
What will be an ideal response?
What diagnostic equipment is usually needed to diagnose faults or relearn the electric power steering system?
A) A breakout box B) Factory of factory-level scan tool C) Special electronic diagnostic equipment designed to test each specific system D) A 12-volt test light
Which of the following filters has the important property that its impulse response rings at the symbol rate, which means that it is capable of minimizing ISI?
A. Bandpass B. Low-pass C. Raised-cosine D. Gaussian