Methylcyclopentane (CH3C5H9) will isomerize to cyclohexane (C6H12) at 298 K and 1 bar. Calculate the equilibrium composition of this isomerization reaction. The table below might have useful information. State all assumptions clearly.
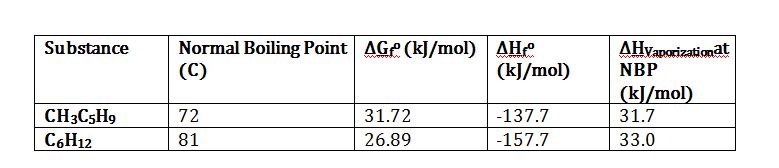
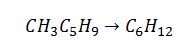
Apply definition of equilibrium constant to solve for K_298K:
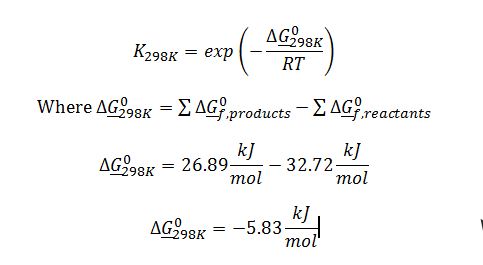
Substitute to solve for K_298K:
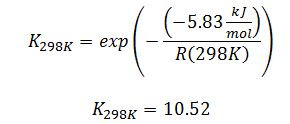
We are well below the normal boiling point so assume we are in the liquid phase. These two compounds are isomers of each other, and both have ringed structures, so it is reasonable to assume that they form ideal liquid solutions:
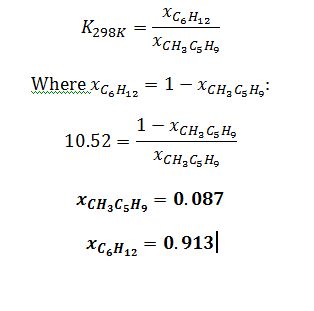
You might also like to view...
In the creation of an offset section view, the transitions between the various cutting segments are known as ____.
A. step segments B. cutting planes C. offset segments D. half section
Ethics
What will be an ideal response?
_________________________ refers to fertilizer salinity
Fill in the blank(s) with the appropriate word(s).
Modern agriculture could also be called:
a. industrialized agriculture b. conventional agriculture c. high-input agriculture d. All of the above are correct.