Solve the problem.The pressure of a gas varies jointly as the amount of the gas (measured in moles) and the temperature and inversely as the volume of the gas. If the pressure is 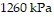 (kiloPascals) when the number of moles is 4, the temperature is
(kiloPascals) when the number of moles is 4, the temperature is 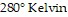 , and the volume is
, and the volume is 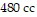 , find the pressure when the number of moles is 6, the temperature is
, find the pressure when the number of moles is 6, the temperature is  , and the
, and the
volume is 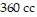 .
.
A. 1215
B. 2250
C. 2340
D. 1170
Answer: C
You might also like to view...
Provide an appropriate response.Find angle A. Round to the nearest 0.1°. 
A. 27.9° B. 116.4° C. 35.7° D. 144.3°
Change the measurements on this scale drawing to decimals and round them to the nearest tenth of an inch. src="https://sciemce.com/media/4/ppg__tttt0618191158__f1q39g6.jpg" style="vertical-align: -17.0px;" /> in. length a is:
A. 4.7 in.
B. 4.4 in.
C. 4.5 in.
D. 4.6 in.
 a 4
a 4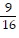 in. b
in. b 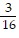 in. c
in. c  in. d
in. d  in. e 2
in. e 2
Determine which of the following methods is the best choice for solving the given equation: factoring, using the principle of square roots or using the quadratic formula. Then, solve the equation.x2 + 4x - 12 = 0
A. Quadratic formula; -2 ± i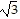
B. Square root principle or factoring; 6, 2
C. Square root principle; -6 ± 2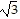
D. Factoring; -6, 2
Match the equation with its graph.(x + 3)2 + (y - 3)2 = 9
A. 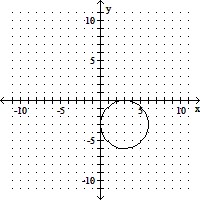
B. 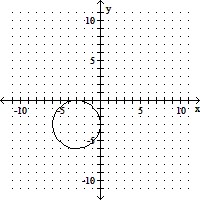
C. 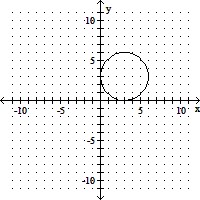
D. 