An ideal gas at pressure, volume, and temperature, P0, V0, and T0, respectively, is heated to point A, allowed to expand to point B also at A’s temperature 2T0, and then returned to the original condition. The internal energy decreases by 3P0V0/2 going from point B to point
T0. How much heat left the gas from point B to point T0?
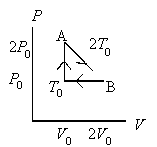
a.
0
b.
P0V0/2
c.
3P0V0/2
d.
5P0V0/2
d
You might also like to view...
A 3.00 kg mass and a 4.00 kg mass are released simultaneously. What is the velocity of the center of mass at t = 10 s (ignore air resistance)?
A. 9.8 m/s B. 7.0 m/s C. 70 m/s D. 98 m/s
When light from air passes through a prism, light of all frequencies
A) slow down. B) remain at speed c. C) speed up. D) none of the above
A chemical reaction is at equilibrium when
a. no reactants are changing to products. b. the forward and reverse reactions proceed at the same rate. c. the total mass has become constant. d. the temperature is no longer changing.
The part of the mantle located directly below the lithosphere is called the ______________
Fill in the blank(s) with correct word