Superheated ammonia enters a mixing chamber at a pressure of 50 psia at a mass flow rate of 5 lbm/s. Saturated liquid ammonia enters the chamber at a rate of 0.60 lbm/s, at 50 psia. Heat is lost from the chamber at a rate of 125 Btu/s. If the combined exit flow is saturated vapor ammonia at 50 psia, determine the temperature of the superheated ammonia which entered the chamber.
State 1: Vapor inlet; State 2: Saturated liquid inlet; State 3: Outlet
Given: P1 = P2 = P3 = 50 psia; m?1= 5.0 lbm/s; x2 = 0.0; m?2=0.60lbms; x3 = 1.0; Q?=?125 Btu/s
Assume: Given no other information regarding the mixing chamber, make the following common assumptions: W?=?KE=?PE=0
Also, assume the mixing chamber is a multiple-inlet, single-outlet, steady-state, steady-flow device.
What will be an ideal response?
The First Law for Open Systems reduces to Q?+m?1h1+m?2h2=m?3h3
The conservation of mass yields: m?1+m?2=m?3 = 5.6 lbm/s
For ammonia:
h2 = 66.0 Btu/lbm
h3 = 617.6 Btu/lbm
Solving the First Law for h1: h1=m?3h3?m?2h2?Q?m?1 = 632.9 Btu/lbm
At P1, this enthalpy value corresponds to T1 = 47.2°F
You might also like to view...
What is the first step usually specified by the vehicle manufacturers when removing an automatic transmission (rear-wheel-drive) from the vehicle?
A) Disconnect the negative battery cable B) Remove the driveshaft C) Hoist the vehicle D) Remove the torque converter
List two industrial applications of the synchronous motor.
What will be an ideal response?
Ridge vents on a hip joint should end _____ above the eaves
a. 12" b. 20" c. 24" d. 30"
Use Data Sheet 5. Refer to Figure. Assume 2" threaded pipe. Find c-c of pipe L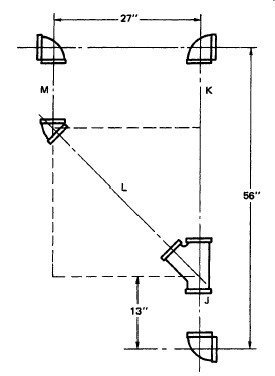
Fill in the blank(s) with the appropriate word(s).