The heat transfer coefficients for the flow of 26.6°C air over a 1.25 cm diameter sphere are measured by observing the temperature-time history of a copper ball of the same dimension. The temperature of the copper ball (c = 376 J/(kg K), ? = 8928 kg/m3) was measured by two thermocouples, one located in the center, and the other near the surface. The two thermocouples registered, within the accuracy of the recording instruments, the same temperature at any given instant. In one test run, the initial temperature of the ball was 66°C and the temperature decreased by 7°C in 1.15 min. Calculate the heat transfer coefficient for this case.
IVEN
• A copper ball with air flowing over it
• Ball diameter (D) = 1.25 cm = 0.0125 m
• Air temperature (T?) = 26.6°C
• Specific heat of ball (c) = 376 J/(kg K)
• Density of the ball (P) = 8928 kg/m3
• Thermocouples in the center and the surface registered the same temperature
• Initial temperature of the ball (To) = 66°C
• Lapse time = 1.15 min = 69 s
• The temperature decrease (To – Tf) = 7°C
FIND
• The heat transfer coefficient ch
ASSUMPTIONS
• The heat transfer coefficient remains constant during the cooling period.
SKETCH
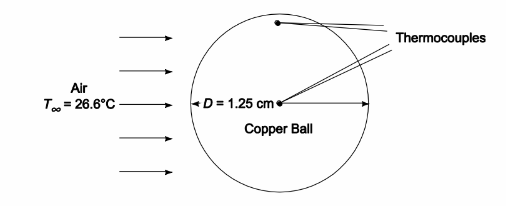
Since the thermocouples register essentially the same temperature, the internal resistance of the ball is small
compared to the external resistance and the ball can be treated with the lumped heat capacity method.
the temperature-time history is
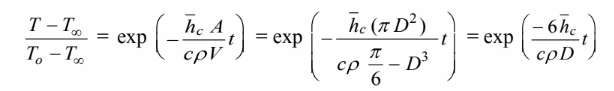
Solving for the heat transfer coefficient
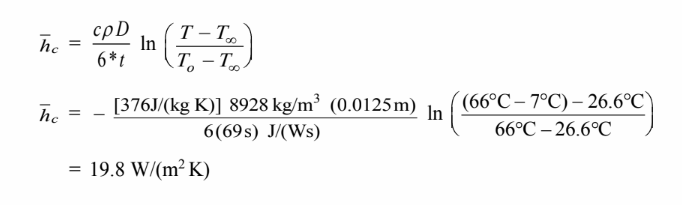
COMMENTS
The value is an average over the cooling period.
The procedure described by this problem can be used to evaluate heat transfer coefficients for odd shaped
object experimentally.
You might also like to view...
Which of these has the greatest number of protons in its nucleus?
A) gold B) mercury C) lead D) silver
One important feature of the Carnot cycle is that it
A. predicts the maximum efficiency of a heat engine operating between two temperatures. B. maximizes the entropy of a heat engine operating between two temperatures. C. specifies the operating temperatures of any heat engine. D. converts all of the heat flowing into an engine to work.
Plants get their energy directly from the Sun and their carbon from the atmosphere. Given this, they can be classified metabolically as
A) photoheterotrophs B) chemoheterotrophs C) chemoautotrophs D) photoautotrophs
Why aren't small asteroids spherical in shape?
A) Small asteroids have odd shapes because they were all chipped off larger objects. B) Large asteroids became spherical because many small collisions chipped off pieces until only a sphere was left; this did not occur with small asteroids. C) The strength of gravity on small asteroids is less than the strength of the rock. D) Large asteroids were once molten and therefore became spherical, but small asteroids were never molten.