Solve.The process by which a gas escapes from a container with a small puncture is called "effusion". Under identical conditions, gases with relatively small particle masses effuse more rapidly than gases with larger particle masses. The relative rate of effusion of helium (He) and nitrogen (N2) can be computed using: relative rate = 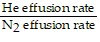 =
= 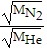 ,where MHe is the mass of the helium particles and MN2 is the mass of the nitrogen particles. Assuming MHe = 4 and MN2 = 28 (arbitrary units), find the exact value of the relative rate of effusion.
,where MHe is the mass of the helium particles and MN2 is the mass of the nitrogen particles. Assuming MHe = 4 and MN2 = 28 (arbitrary units), find the exact value of the relative rate of effusion.
A. 
B. 7
C. 
D. 
Answer: C
Mathematics
You might also like to view...
Perform the indicated operation
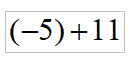
Mathematics
Solve the system by the addition method. 2x + 10y = -5610x + 2y = 56
A. {(-7, 7)} B. {(-2, 7)} C. {(7, -7)} D. {(10, -10)}
Mathematics
Solve the problem.An airplane has an air speed of 550 miles per hour bearing N30°W. The wind velocity is 50 miles per hour in the direction N30°E. To the nearest tenth, what is the ground speed of the plane? What is its direction?
A. 552.3 mph; N24.8°W B. 552.3 mph; N54.8°W C. 576.6 mph; N25.7°W D. 526.8 mph; N55.3°W
Mathematics
Solve.x2 = 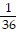
A.  , -
, - 
B.  , -
, - 
C. 
D. 
Mathematics