First Law of Thermodynamics: The figure shows a pV diagram of a gas for a complete cycle. During part bc of the cycle, 1190 J of heat flows into a system, and at the same time the system expands against a constant external pressure of 7.00 × 104 Pa as its volume increases from 0.0200 m3 to 0.0800 m3. Calculate the change in internal (thermal) energy of the system during part bc of the cycle. If the change is nonzero, be sure to indicate whether the change is positive or negative.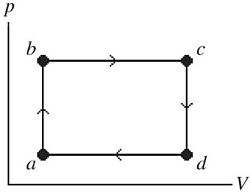
Fill in the blank(s) with the appropriate word(s).
-3010 J
You might also like to view...
Weight and g: An piece of space debris is released from rest at an altitude that is two earth radii from the center of the earth. Compared to its weight on Earth, the weight of this debris is
A. zero. B. the same as on the surface of the earth. C. one-half of its weight on the surface of the earth. D. one-third of its weight on the surface of the earth. E. one-quarter of its weight on the surface of the earth.
At which wavelength range is there no current or planned space observatory?
A) radio B) infrared C) visible D) X-ray E) gamma-ray
The Great Red Spot on Jupiter is ________.
A. a largo tornado sweeping the planet from north to south B. a large rising cloud of gas C. a planetesimal impact site D. a giant vortex that has persisted for over 300 years
At t = 0 the switch S is closed with the capacitor uncharged. If C = 30 ?F, ? = 30 V, and R = 5.0 k?, at what rate is energy being stored in the capacitor when I = 2.0 mA?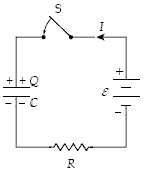
A. 32 mW B. 40 mW C. 44 mW D. 36 mW E. 80 mW