Solve the problem.The volume V (in liters) of 1 mole of a gas is related to its temperature T (in Kelvin) and pressure P (in atmospheres) by van der Waals' equation 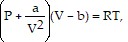 where the constant R is 0.08207. For krypton,
where the constant R is 0.08207. For krypton, 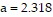 and
and 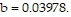 Use Newton's method to find the volume V of 1 mole of krypton if
Use Newton's method to find the volume V of 1 mole of krypton if 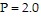 atmospheres and
atmospheres and
src="https://sciemce.com/media/4/ppg__efdesf0601192012__f1q28g5.jpg" alt="" style="vertical-align: -4.0px;" /> Round your answer to two decimal places.
A. 17.61 L
B. 12.53 L
C. 15.49 L
D. 13.50 L
Answer: D
Mathematics
src="https://sciemce.com/media/4/ppg__efdesf0601192012__f1q28g5.jpg" alt="" style="vertical-align: -4.0px;" /> Round your answer to two decimal places.
A. 17.61 L
B. 12.53 L
C. 15.49 L
D. 13.50 L
Answer: D
You might also like to view...
Compute the gradient of the function at the given point.
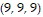
A. ?f = 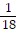 i +
i +  j -
j -  k
k
B. ?f = - 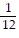 i +
i + 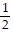 j -
j - 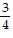 k
k
C. ?f = - 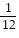 i +
i + 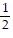 j -
j - 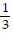 k
k
D. ?f = 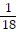 i +
i + 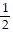 j -
j - 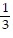 k
k
Decide whether or not the events are mutually exclusive.Events A and B defined as followsEvent A is that at least three of Toni's five cousins are female. Event B is that at most four of Toni's five cousins are male.
A. Yes B. No
Solve the problem.Write an equation for the tangent line to the curve 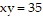 at the point
at the point 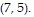
A. 5x + 7y = 70 B. 5x + 7y = 35 C. 7x + 5y = 70 D. 7x + 5y = 35
Perform the indicated operation.2.2 km + 358 m (in kilometers)
A. 5.78 km B. 2.558 km C. 358,002.2 km D. 2.2358 km