When a sample of water at 0.0°C is cooled to –36.0°C and freezes in the process, 935,000 kJ of heat is liberated
What is the mass of this sample of water? For water LF = 334,000 J/kg, LV = 2.256 × 106 J/kg, and the specific heat of ice is 2050 J/kg ? C°.
A) 2290 kg
B) 1145 kg
C) 2800 kg
D) 12,700 kg
A
You might also like to view...
How many valence electrons does boron (B, atomic no. = 5) have?
A) 1 B) 2 C) 3 D) 4 E) 5
Before the switch is closed in the figure, the potential across the capacitor is 200 V. At some instant after the switch is closed, the instantaneous current is 0.70 A. What is the energy in the capacitor at this instant?
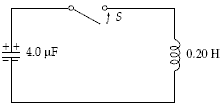
a.
49 mJ
b.
31 mJ
c.
80 mJ
d.
0.13 J
e.
62 mJ
Two long, parallel wires carry currents of 4.00 A and 6.00 A. If the distance between the wires is 0.400 m, what is the force per unit length between the wires?
A) 2.00 ?N/m B) 5.00 ?N/m C) 12.0 ?N/m D) 16.0 ?N/m E) 38.0 ?N/m
The nova event is created by the helium flash
Indicate whether the statement is true or false