Which estimate do you believe is the most accurate and why?
Estimate the molar volume (in cm3/mol) of superheated butane at 393.2 K and 38 bar using the following methods:
A) Ideal Gas Law.
B) Using Virial EOS (Z = 1 + BP/R/T)…getting B from correlations
C) Using Pitzer expression for Z using the Lee-Kessler approach
A) Using the Ideal Gas equation:
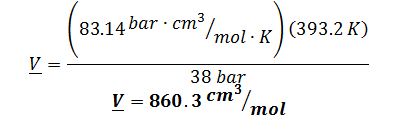

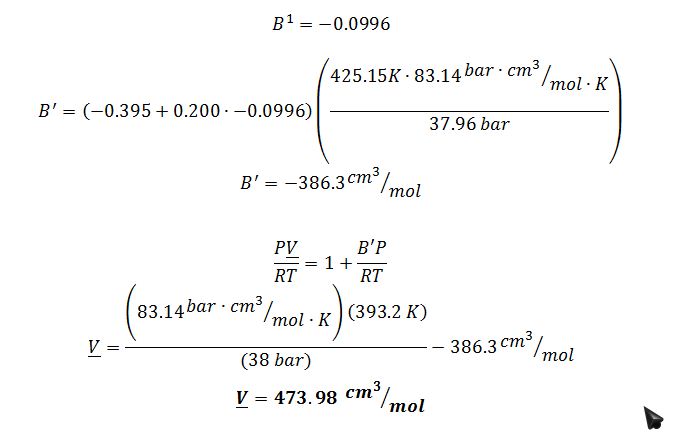
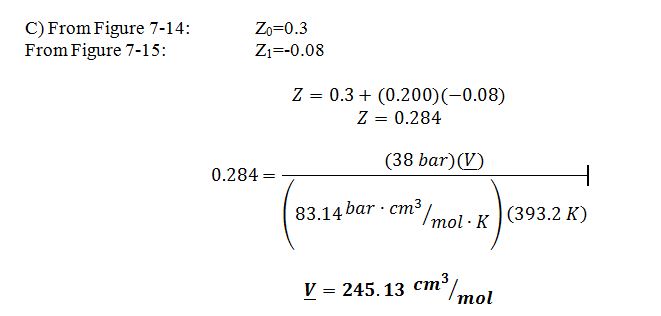
The best estimate would be the Lee-Kesler method. Ideal Gas is not a good model because the butane is a high pressure. Virial is not a good estimate because these conditions for butane are not in the linear region of the model, as indicated by the check below:
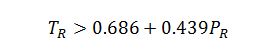
However, for butane at these conditions:
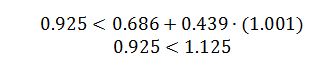
You might also like to view...
Compute the volume in cubic inches of a sphere whose diameter is 9.2 inches. Round to one decimal place.
A. 270.1 B. 407.7 C. 385.4 D. 3261.8
A compressive force is applied to a structure, the strain = 5 micro strains. Two separate strain gauges are attached to the structures, one is a nickel wire strain gauge of gauge factor = –12.1 and the other is a nicrome wire strain gauge of gauge factor = 2. Calculate the value of resistance of the gauges after they are strained. The resistance of strain gauge =120 ohms.
What will be an ideal response?
An RC low-pass filter attenuates the output the most at
A. medium frequencies B. high frequencies C. no frequencies (there is no attentuation whatsoever at any frequency) D. low frequencies
One of the most popular applications of a potentiometer is as an adjustable voltage divider also known as a:
A) volume control. B) current control. C) divider control. D) voltage control.