Light shines through atomic hydrogen gas. It is seen that the gas absorbs light readily at a wavelength of 91.63 nm
What is the value of n of the level to which the hydrogen is being excited by the absorption of light of this wavelength? Assume that the most of the atoms in the gas are in the lowest level. (h = 6.626 × 10-34 J ? s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J, the Rydberg constant is R = 1.097 × 107 m-1) A) 14
B) 16
C) 11
D) 21
A
You might also like to view...
Molar Specific Heats: A certain ideal gas has a molar specific heat at constant pressure of 33.2 J/mol ? K. Its molar specific heat at constant volume is closest to which of the following values? (R = 8.31J/mol ? K)
A. 41.9 J/mol ? K B. 16.6 J/mol ? K C. 25.1 J/mol ? K D. 24.9 J/mol ? K E. 49.8 J/mol ? K
A parallel plate capacitor with plate separation of 4.0 cm has a plate area of 4.0 × 10-2 m2. What is the capacitance of this capacitor with air between these plates?
A) 8.9 × 10-11 F B) 8.9 × 10-12 F C) 8.9 × 10-13 F D) 8.9 × 10-14 F E) 8.9 × 10-15 F
A proton is an example of
A) a lepton. B) a meson. C) a baryon. D) a gauge boson. E) none of the above.
The figures below represent interference fringes. The distances from the screen to the slits is the same for each figure, and the planes of the screen and the slits are parallel. Which figure(s) represent(s) slits with the smallest spacing d between the slits? The white spaces represent the interference maxima
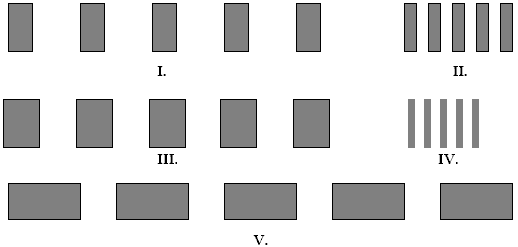
a.
I.
b.
II.
c.
III.
d.
IV.
e.
V.