First Law of Thermodynamics: A cyclic process is carried out on an ideal gas such that it returns to its initial state at the end of a cycle, as shown in the pV diagram in the figure. If the process is carried out in a clockwise sense around the enclosed area, as shown on the figure, then the magnitude of the enclosed area represents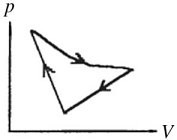
A. the heat that flows out of the gas.
B. the work done on the gas.
C. the heat added to the gas.
D. the work done by the gas.
Answer: D
You might also like to view...
As an electron moves farther from the nucleus, it ________ energy.
A. loses B. gains C. generates D. absorbs E. quantizes
Refer to the diagram of Racetrack X.  This special racetrack is all curve and no straightaway. If a driver takes her car around this track counterclockwise and at constant speed, then greater acceleration will occur at any place the turning radius is
This special racetrack is all curve and no straightaway. If a driver takes her car around this track counterclockwise and at constant speed, then greater acceleration will occur at any place the turning radius is
A. larger. B. inward. C. outward. D. smaller.
If the accuracy in measuring the position of a particle increases, the accuracy in measuring its velocity will
A) increase. B) decrease. C) remain the same. D) It is impossible to say since the two measurements are independent and do not affect each other.
The numerals that follow the symbols in chemical formulas are called ______________
Fill in the blank(s) with correct word