The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water?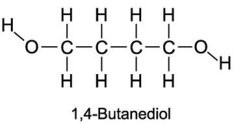
A. Since there are no polar areas on this molecule, it is insoluble in water at room temperature.
B. Since there are polar areas on this molecule, it is insoluble in water at room temperature.
C. Water would be attracted to both ends of 1,4 butanediol, and it is infinitely soluble in water.
D. A high boiling point means that the substance interacts with itself quite strongly. Therefore, this molecule is not soluble in water.
Answer: C
You might also like to view...
A(n) ____________________ reaction is any chemical reaction activated by light.
Fill in the blank(s) with the appropriate word(s).
Which category of toxic chemical has an acute response that includes death?
A. neurotoxin B. carcinogen C. teratogen D. mutagen
When minerals replace the bodily remains during fossilization, we say the fossil has been ____
a. molded b. dissolved c. altered d. indexed e. regurgitated
The leading cause for migration to the United States and Canada has been
A) freedom from religious persecution. B) avoiding harsh environmental conditions. C) forced migration. D) economic opportunity. E) fleeing dictatorial governments.