In an adiabatic process (one in which no heat transfer takes place), the pressure P and volume V of an ideal gas such as oxygen satisfy the equation 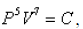 where C is a constant. Suppose that at a certain instant of time, the volume of the gas is 2L, the pressure is 100 kPa, and the pressure is decreasing at the rate of 5 kPa/sec. Find the rate at which the volume is changing.
where C is a constant. Suppose that at a certain instant of time, the volume of the gas is 2L, the pressure is 100 kPa, and the pressure is decreasing at the rate of 5 kPa/sec. Find the rate at which the volume is changing.
What will be an ideal response?
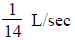
You might also like to view...
Determine the limit by sketching an appropriate graph.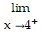 f(x), where f(x) =
f(x), where f(x) = 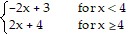
A. 5 B. 4 C. -5 D. 12
Find the probability.The participants in a television quiz show are picked from a large pool of applicants with approximately equal numbers of men and women. Among the last 11 participants there have been only 2 women. If participants are picked randomly, what is the probability of getting 2 or fewer women when 11 people are picked?
A. 0.0327 B. 0.0059 C. 0.0269 D. 0.0322
Use the Gauss-Jordan method to solve the system of equations. If the system has infinitely many solutions, give the solution with y arbitrary.9x - 7y = -14-7x + 4y = 8
A. ? B. {(-1, 3)} C. {(0, 2)} D. {(0, 3)}
Provide an appropriate response.Determine if the expression (-3)-2 is positive or negative.
A. Positive B. Negative