A monatomic ideal gas undergoes an isothermal expansion at 300 K, as the volume increased from 0.03 m3 to 0.21 m3. The final pressure of the gas is 60 kPA The ideal gas constant is R = 8.314 J/mol ? K
The change in the internal (thermal) energy of the gas is closest to A) 0.00 kJ.
B) 12 kJ.
C) 25 kJ.
D) -12 kJ.
E) -25 kJ.
A
You might also like to view...
A sealed 26- tank is filled with 2000 moles of oxygen gas (O2) at an initial temperature of 270 K
The gas is heated to a final temperature of 460 K. The ATOMIC mass of oxygen is 16.0 g/mol, and the ideal gas constant is R = 8.314 J/mol · K = 0.0821 L · atm/mol · K. The final pressure of the gas is closest to A) 0.29 MPa. B) 0.31 MPa. C) 0.33 MPa. D) 0.34 MPa. E) 0.36 MPa.
Calculate Vout/Vin for the circuit if R = 2.0 k?, C = 0.020 ?F and V = 140 V sin(50,000t).
?
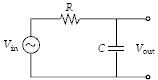
A. 0.02 B. 0.45 C. 0.80 D. 0.98 E. 2.2
An empty jug of weight W is at rest on a table. When water of weight w is poured into it, the amount of support force supplied by the table is
A) W. B) W + w. C) W - w. D) none of the above
In a fission reaction, a 235U nucleus captures a neutron. What energy is released if the products are 139I, 95Y and two neutrons? (atomic masses: 235U, 235.043 9; 139I, 138.935 0; 95Y, 94.913 4; neutron, 1.008 67; and 1 u = 931.5 MeV/c2)
a. 123 MeV b. 174 MeV c. 199 MeV d. 218 MeV