One kilogram of air is heated reversibly at constant pressure from an initial state of 300 K and 1 bar until its volume triples. Calculate W, Q, ?U, and ?H for the process. Assume for air that PV / T = 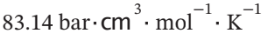 and
and 
What will be an ideal response?
If PV/T is constant then at constant pressure, the volume will triple when the temperature triples, so the final state will be 900 K and 1 bar. The enthalpy change for this constant volume process with constant heat capacity is given by 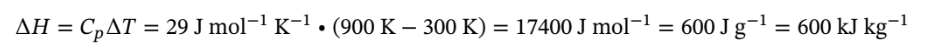
where we've used the molecular weight of air of about 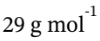
to convert from molar enthalpy change to specific enthalpy change.
By the definition of U, we have
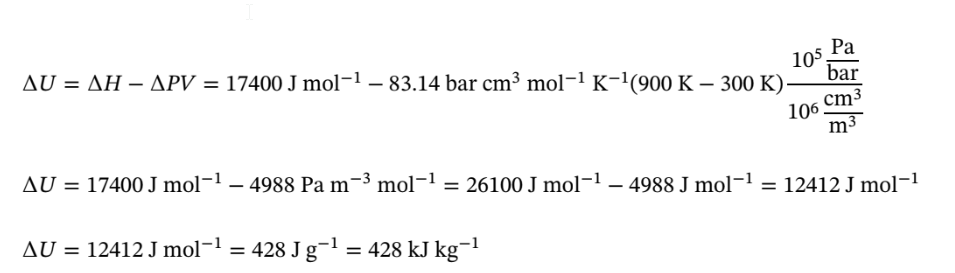
The work is given by –P?V = –?PV at constant P, which we just computed to be
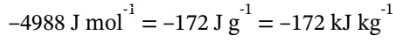 . For a constant pressure process, Q is equal to the enthalpy change, so Q = 600 kJ/kg
. For a constant pressure process, Q is equal to the enthalpy change, so Q = 600 kJ/kg
You might also like to view...
Which type of fifth wheel is most commonly used on highway tractors?
A. Fully oscillating B. Semi-oscillating C. Stabilized D. Elevating
A construction company can purchase a used backhoe for $90,000 and spend $450 per day in operating costs. The equipment will have a 5-year life with no salvage value. Alternatively, the company can lease the equipment for $800 per day. How many days per year must the company use the equipment in order to justify its purchase at an interest rate of 8% per year.
What will be an ideal response?
What could cause ice to build up in the ice bin?
What will be an ideal response?
A metal mesh sling should be discarded if abrasion has caused a reduction of wire diameter of _____
a. 5 percent b. 10 percent c. 25 percent d. 30 percent