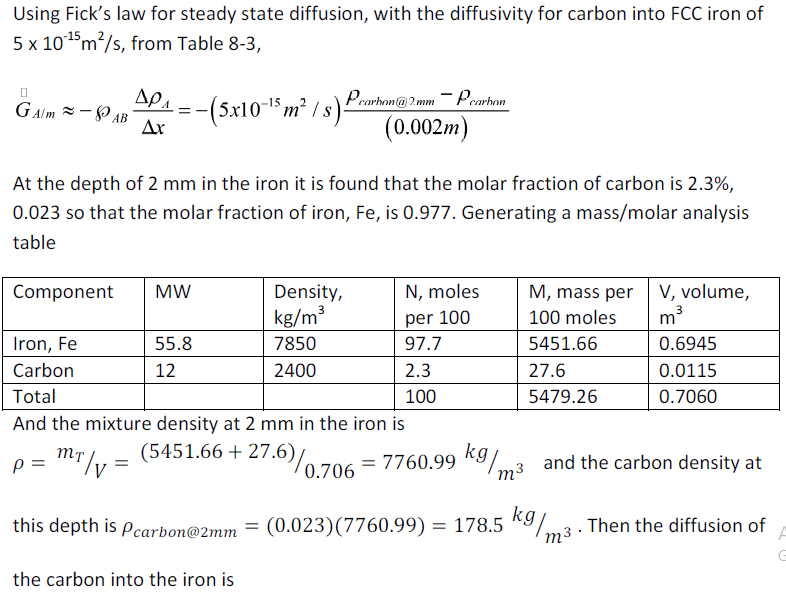
Carburizing iron is a process to increase its strength by diffusing carbon into the metal. If the process carried out at 500ºC, estimate the mass diffusion rate into the iron when the carbon concentration is 2.3% (by volume) at a depth of 2 mm. Assume that the iron is face-centered cubic (FCC) and the carbon concentration at the surface is 100%.
What will be an ideal response?
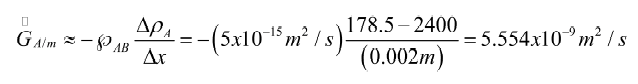
Physics & Space Science
You might also like to view...
Ionization from the ____________________ star in the Orion nebula makes it ____________________ brightly
Fill in the blank(s) with correct word
Physics & Space Science
The position of an object that is oscillating on an ideal spring is given by the equation x = (12.3 cm) cos[(1.26s-1)t]. At time t = 0.815 s,
(a) how fast is the object moving? (b) what is the magnitude of the acceleration of the object? What will be an ideal response?
Physics & Space Science
How much better resolution would a 60 mm objective lens have than your eye's 6 mm exit pupil?
A) 6 times B) 10 times C) 16 times D) 60 times E) 100 times
Physics & Space Science
which of the following stars lives the longest life?
What will be an ideal response?
Physics & Space Science