Solve the problem.The pH of a chemical solution is given by the formula pH = -log10[H+] where [H+] is the concentration of hydrogen ions in moles per liter. Find the pH if the [H+] = 8.4 x 10-1.
A. 1.92
B. 1.08
C. 0.08
D. 0.92
Answer: C
You might also like to view...
Plot the curve of the given polar equation in polar coordinates.r = 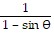
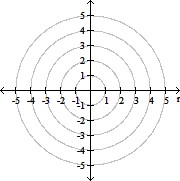
A. 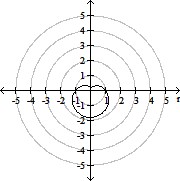
B. 
C. 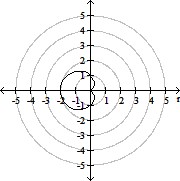
D. 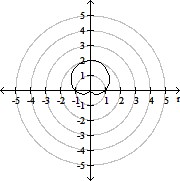
Sketch the graph of the quadratic function. Identify the vertex, intercepts, and the equation for the axis of symmetry.f(x) = 2x2 + 12x + 22
A. vertex (3, 4)
x-intercepts: none
y-intercept (0, 22)
axis of symmetry: x = 3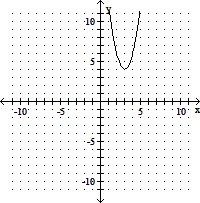
B. vertex (-3, 4)
x-intercepts: none
y-intercept: 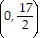
axis of symmetry: x = -3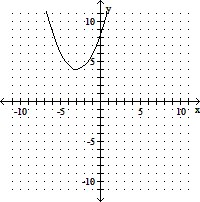
C. vertex (3, 4)
x-intercepts: none
y-intercept 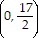
axis of symmetry: x = 3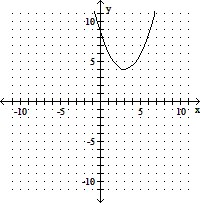
D. vertex: (-3, 4)
x-intercepts: none
y-intercept: (0, 22)
axis of symmetry: x = -3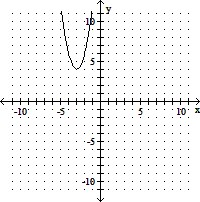
Graph the function.y = -cot (?x)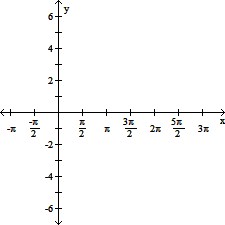
A. 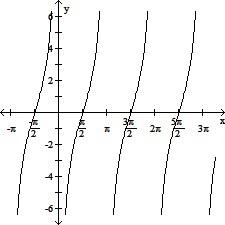
B. 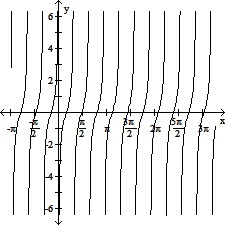
C. 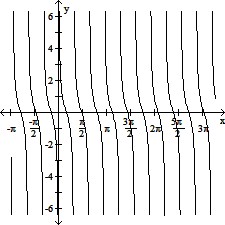
D. 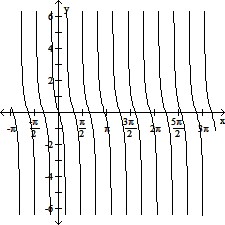
Find the area of a sector of a circle having radius r and central angle ?. If necessary, express the answer to the nearest tenth.r = 7.0 mi, ? = 268°
A. 16.4 mi2 B. 38.3 mi2 C. 229.2 mi2 D. 114.6 mi2