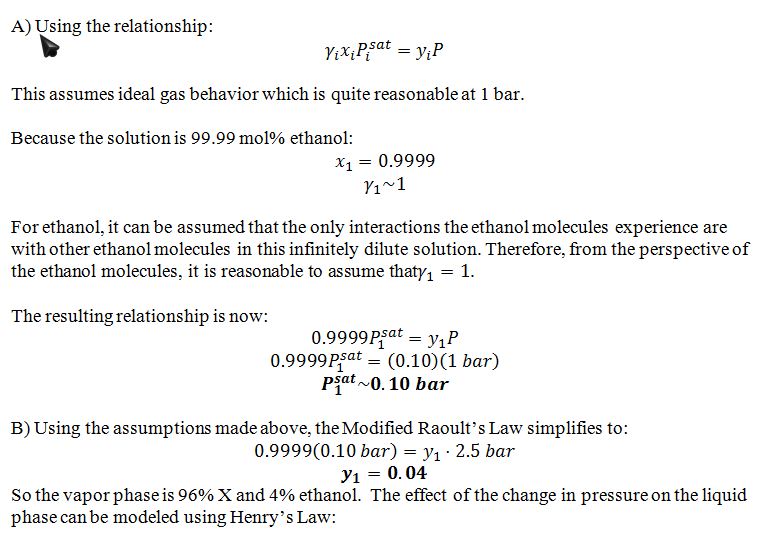
At T=25 ?C, pure compound X has a large vapor pressure. However, the X is also slightly soluble in liquid ethanol. Consequently, when X vapor is bubbled through pure liquid ethanol at P=1 bar and T=25 ?C, a small amount of X dissolves in the liquid, and in addition, some ethanol evaporates into the vapor phase.
At equilibrium at P=1 bar and T=25 ?C, the liquid phase contains 99.99 mol% ethanol and 0.01 mol% X, and the vapor leaving the bubbler contains 10 mol% ethanol and 90 mol% X.
A) Based only on the given information, give your best estimate of the vapor pressure of ethanol at 25 ?C. List any assumptions you made to solve the problem and assess each for this system. If you say it is reasonable, explain why, and if you say it is not reasonable, then list the additional information you would need to perform a more accurate calculation.
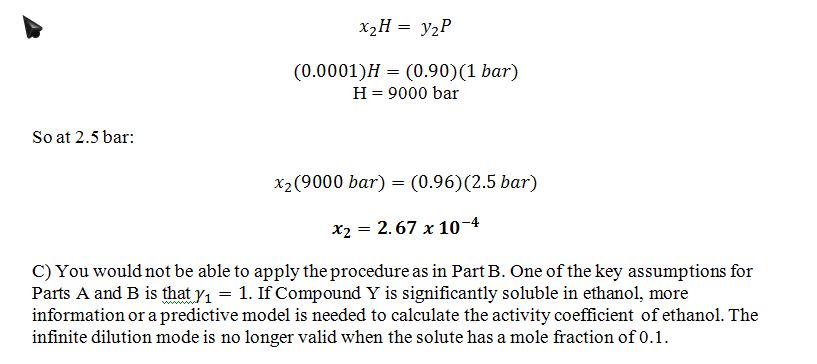
B) The pressure in the bubbler is increased from 1 bar to 2.5 bar. Give your best estimates of the new equilibrium compositions of the liquid and vapor phases.
C) There is a different compound called Y, which also has a large vapor pressure, but it is a lot more soluble in ethanol than X. In fact, when pure Y is bubbled through pure liquid ethanol at 25 ?C and 1 bar, the liquid phase at equilibrium contains 10 mol% Y and 90 mol% ethanol. If you wanted to calculate the effect of increasing the pressure to 2.5 bar for this system, would it be reasonable to apply the same solution procedure you used in part B? Why or why not?
You might also like to view...
On the Conservation Philosophy Continuum, what kind of person advocates keeping something the way it is or returning
it to its natural state?
What will be an ideal response?Human saliva contains the enzyme ____________________, which initiates the digestion of starch
Fill in the blank(s) with correct word
If an electrician works ![]() hours a day on a job at $16 per hour; how much money does he earn in 5 days?
hours a day on a job at $16 per hour; how much money does he earn in 5 days?
Fill in the blank(s) with the appropriate word(s).
How is the concentration of a commodity defined? How is the concentration gradient defined? How is the diffusion rate of a commodity related to the concentration gradient?
What will be an ideal response?