An ideal gas has a pressure of 2.5 atm, a volume of 1.0 L at a temperature of 30°C. How many molecules are there in this gas? (R = 8.31 J/mol ? K,1.00 atm = 101 kPa, NA = 6.022 × 1023)
A) 2.4 × 1022 B) 6.1 × 1023 C) 2.3 × 1023 D) 6.0 × 1022
D
You might also like to view...
Thermodynamic Processes: The figure shows a pV diagram for 0.98 mol of ideal gas that undergoes the process 1 ? 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? (R = 8.31 J/mol ? K).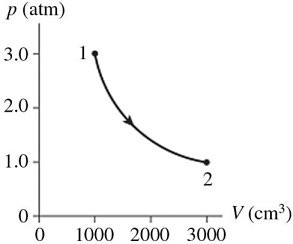
A. -160°C B. 12°C C. 380°C D. 110°C
In about 14,000 AD, the North Pole of Earth will point at star ________.
A. Antares B. Vega C. Thuban D. Polaris
A slab of material 1 rests on the surface of a slab of material 2 . A light ray in material 1 has an angle of incidence of 30° on the interface between the materials, while the ray in material 2 has an angle of refraction of 45°. What is the critical angle of incidence for total internal reflection for a ray of light going from material 2 to material 1?
a. 30° b. 45° c. In this case there is no critical angle. d. At least one of the indices of refraction or its equivalent is needed to solve this problem.
The energy used by a 100 watt light bulb in 1 hour is 100 kilowatt-hours
Indicate whether the statement is true or false