An ideal gas is at a temperature of 30 and a pressure of 2.00 atmospheres. What is the number of particles per unit volume of the gas?
and a pressure of 2.00 atmospheres. What is the number of particles per unit volume of the gas?
a. 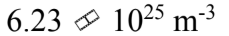
b. 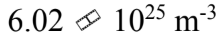
c. 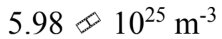
d. 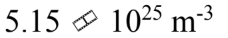
e. 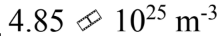
e. 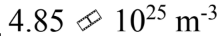
You might also like to view...
Special relativity predicts that the observed mass of a moving particle depends on its ____
a. inertia b. curvature c. velocity d. force e. true position
A depth change of 10 metres/33 feet causes a pressure change of.
a. 1 bar/ata b. 2 bar/ata c. 3 bar/ata d. 4 bar/ata
How could the distance measurements based on white dwarf supernovae be independently validated (or invalidated)?
What will be an ideal response?
Carbon-14 has a half-life of 5730 y. A sample of wood has been recovered by an archaeologist. The sample is sent to a laboratory,
where it is determined that the activity of the sample is 0.144 Bq/g. By comparing this activity with the activity of living organic matter, 0.230 Bq/g, the scientist determines how old the wood sample is, or more precisely, when the tree that the sample came from died. How old is the sample of wood? A) 2940 y B) 3870 y C) 2630 y D) 4250 y E) 4590 y