In a chemical reaction run at a certain temperature, the concentration C of a certain reactant at time t is given by 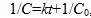 where
where 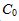 is the initial concentration and k is the rate constant. Assume the initial concentration is known to be 0.04 mol/L exactly. Assume that time is measured with negligible uncertainty.
is the initial concentration and k is the rate constant. Assume the initial concentration is known to be 0.04 mol/L exactly. Assume that time is measured with negligible uncertainty.
a. After 30 s, the concentration C is measured to be 0.0038 ± 2.0 × 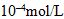 . Estimate the rate constant k, and find the relative uncertainty in the estimate.
. Estimate the rate constant k, and find the relative uncertainty in the estimate.
b. After 50 s, the concentration C is measured to be 0.0024 ± 2.0 ×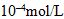 . Estimate the rate constant k and find the relative uncertainty in the estimate.
. Estimate the rate constant k and find the relative uncertainty in the estimate.
c. Denote the estimates of the rate constant k in parts (a) and (b) by 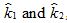 respectively. The geometric mean
respectively. The geometric mean 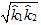 is used as an estimate of k. Find the relative uncertainty in this estimate.
is used as an estimate of k. Find the relative uncertainty in this estimate.
You might also like to view...
Which of the following qualifies as an assembly occupancy?
a. a restroom of a convention center b. the auditorium of a civic center c. a small office in a chamber of commerce d. the walk-in cooler of a cafeteria
Name the first welding symbol element to be located on the drawing, and has additional information is placed to continue the weld specification.
What will be an ideal response?
Approximately what percentage of total freight volume is handled by trucks nationwide?
A. 10% B. 50% C. 80% D. 95%
If two power-side insulated wires were to melt together to the point that the copper conductors touched each other, the type of failure would be called a(an) ______________
A) Short-to-voltage B) Short-to-ground C) Open D) Floating ground