Because glacial ice is a natural, consolidated aggregate of minerals, it is considered to be a rock. Explain what type of rock it is: igneous, metamorphic, or sedimentary
What will be an ideal response?
Glacial ice is a metamorphic rock because it forms from the recrystallization of snow due to increasing pressure with burial beneath the glacier surface.
You might also like to view...
Light nuclei can be split. For example, a deuteron, which is a proton-neutron combination, can split into a separate proton and separate neutron. Does such a process yield energy or cost energy? Why?
A. Costs energy. This fact is consistent with the sum of the masses of the separated proton and neutron being greater than the mass of the original deuteron. B. Neither. The amount of energy required to split the light nuclei is exactly the same as that released. This is the basis of the Law of Conservation of Energy. C. Yields energy. This fact is consistent with the sum of the masses of the separated proton and neutron being less than the mass of the original deuteron. D. Yields energy. Splitting nuclei of any sort is a basic example of a nuclear reaction releasing massive amounts of energy.
Locate and give the geographic coordinates for the following cities (to a tenth of a degree if your atlas maps are detailed enough) or identify the cities from the given coordinates. The answers to a) and e) are provided for you in bracketed italics. Once you have identified the cities and found the coordinates, plot the coordinates in items 1 (a) through (h) above on the map grid in Figure 2.1, and label the city names.
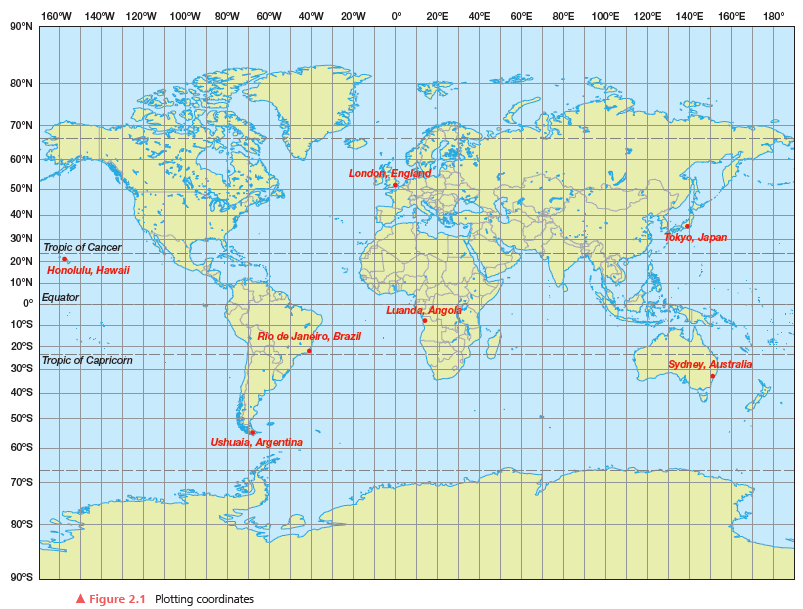
City
(a) Greenwich, London, England
(b) Rio de Janeiro, Brazil
(c) Sydney, Australia
(d) __________________________
(e) __________________________
(f) __________________________
(g) __________________________
Latitude and Longitude
(a) __________________________
(b) __________________________
(c) __________________________
(d) 35.7°N 139.7°E
(e) 8.8°S 13.2°E
(f) 21.3°N, 157.8°W
(g) 54.8°S, 68.3°W
The fact that life expectancy has increased significantly over the past 60 years in developing nations is primarily a result of the
A) epidemiologic transition. B) cultural transition. C) demographic transition. D) socioeconomic transition.
When phosphoric acid, H3PO4(aq) , reacts with solid magnesium hydroxide, Mg(OH)2(s), the products are aqueous magnesium phosphate and water. Which of the following is the balanced equation for this reaction?
A) H3PO4(aq) + Mg(OH)2(s) ? MgPO4(aq) + H2O(l) B) 2 H3PO4(aq) + Mg(OH)2(s) ? Mg(PO4)2(aq) + 2 H2O(l) C) 3 H3PO4(aq) + 2 Mg(OH)2(s) ? Mg2(PO4)3(aq) + 2 H2O(l) D) 2 H3PO4(aq) + 3 Mg(OH)2(s) ? Mg3(PO4)2(aq) + 6 H2O(l)