For a substance that has a molar volume of V = kP, k being a constant, and a constant heat capacity, which of these is equal to the Joule-Thompson coefficient?
A. -kP
B. V
C. kP/CP
D. 1/CP
E. None of these
A. Incorrect. Check your definition of Joule-Thomson coefficient.
B. Incorrect. Molar volume has the wrong units for the Joule-Thomson coefficient.
C. Incorrect. Check your signs.
D. Incorrect. Check your definitions.
E. Correct. The correct solution is -kP/CP
You might also like to view...
For continuous random variables X and Ywith joint probability density function
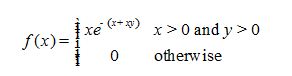
a. Find P(X > 1 and Y > 1).
b. Find the marginal probability density functions 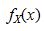 and
and 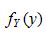
c. Are X and Y independent? Explain.
What is the equivalent impedance, in rectangular form, of the series combination of 1<0°> and 1?
A. 2 + 2j B. 0 C. 2 ? 2j D. 1 ? j E. 1 + j
Conductors in a capacitance device are separated by ________
A) Insulators B) A vacuum C) Conductors D) None of these
What type of fixed resistor is made from a compound of carbon graphite and resin bonding material?
A. composition carbon B. metal film C. metal glaze D. wire-wound