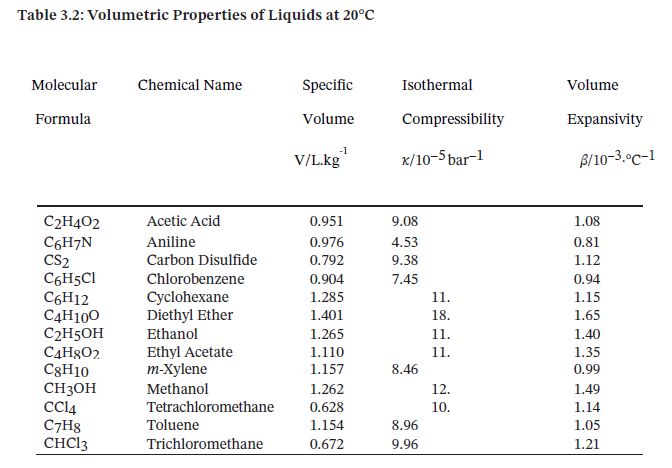
For one of the substances in Table 3.2, compute the final pressure when the substance is heated from 15°C and 1 bar to 25°C at constant volume.
Table 3.2 provides the specific volume, isothermal compressibility, and volume expansivity of several liquids at 20°C and 1 bar25, where ? and ? may be assumed constant.
First ethanol will be chosen as the substance to be used. In the problem the volume is held constant, both the temperature and pressure come into play here. We start with the equation
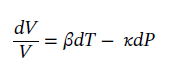
At constant volume, it becomes
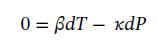
Integrating
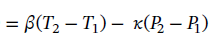
and solving for P2 gives
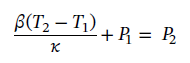
Plugging in the values gives
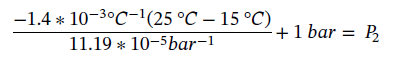
Leading P2 = 126.11 bar
You might also like to view...
?What GMAW process is used to make the welds on the 10 gauge to 14 gauge thick carbon steel joints?
A. ?GMAW-S B. ?GMAW-P C. ?GMAW-G D. ?GMAW-spray metal arc transfer
How did the mainstream liberal Protestant churches lose cultural authority in the 1960s? Why were more conservative evangelical Protestants able to take their place?
What will be an ideal response?
What is the power transformer based on?
A. Current transformation B. Voltage transformation C. Iron-core transformation D. Impedance transformation
The __________ is the difference between the condition of the controlled variable and the set point.
A. measured variable B. feedback signal C. error signal