Calorimetry: A 0.600-kg piece of metal X is heated to 100°C and placed in an aluminum can of mass 0.200-kg which contains 0.500 kg of water initially at 17.3°C. The final equilibrium temperature of the mixture is 20.2°C, what is the specific heat of metal X? The specific heats of water and aluminum are 4186 J/kg ? K (water) and 910 J/kg ? K (aluminum).
A. 140 J/kg ? K
B. 270 J/kg ? K
C. 450 J/kg ? K
D. 900 J/kg ? K
Answer: A
You might also like to view...
Two kilograms of molten copper at 1083ºC are dropped into water at 40ºC. Determine the freezing time for complete solidification of the copper. Assume lumped heat capacity and that the heat of fusion of copper is 204.7 kJ/kg and the convective heat transfer coefficient have is 120 W/m2?K. Hint: Use Equation 10-66 for freezing instead of melting.
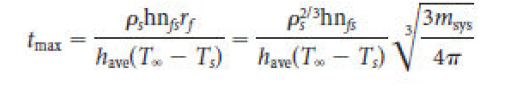
During the nuclear fusion of hydrogen to helium in the core of our Sun, what percentage of the original mass of the hydrogen is converted into energy?
A) 0.7% B) 0.07% C) 70% D) 100%
A 2-kg ball is thrown horizontally at. a speed of 10 m/s. At the same time, a 1-kg ball is dropped from the same height. Ignoring air resistance, which ball hits the ground first?
a. the 2-kg ball b. it's a tie c. the 1-kg ball
Air at 30°C and 200 kPa is flowing in a 30-cm-diameter pipe and passes through the bend shown. An estimate of the pressure difference between the inside of the bend and the outside of the bend is approximately:
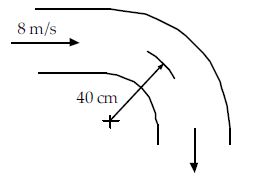
(A) 110 Pa
(B) 140 Pa
(C) 180 Pa
(D) 240 Pa