A gas enters a compressor at T=25 ?C and P=0.2 bar, and exits at T=150 ?C. The compressor operates at steady state, and can be modeled as both adiabatic and reversible. The ideal gas heat capacity of the gas can be considered constant at C_V^*=5R.
A) What is the pressure of the gas leaving the compressor?
B) Suppose the entering pressure had been 5 bar instead of 0.2 bar, but everything else were the same. Discuss which (if any) aspects of the solution to part A would still be applicable, and which aspects (if any) would no longer be applicable.
A) Apply entropy balance for a reversible, adiabatic, steady state compressor:
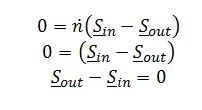
Assume ideal gas behavior since pressure is low @ 0.2 bar. For an ideal gas:
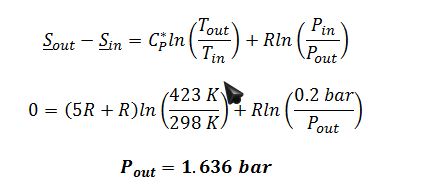
B) If the entering pressure had been 5 bar instead of 0.2 bar, the same calculation would produce an exiting pressure over 40 bar. However, at such conditions it could not be assumed that the gas would act as an ideal gas. Therefore, the ideal gas model for change in entropy of a gas would not be valid in this situation. (Methods of modeling such a process will be covered starting in Chapter 6).
You might also like to view...
Transformers are rated by their primary voltage, secondary voltage, and volt-amperes (VA).
Answer the following statement true (T) or false (F)
What is the treatment for root rot?
What will be an ideal response?
________ studs are the bearing support for wall headers in framed openings
A) Finger jointed B) Jack C) Engineered lumber D) Cripple
Air flow switches are often called ____________________ switches.
Fill in the blank(s) with the appropriate word(s).