A copper cylinder with a mass of 125 g and temperature of 345°C is cooled by dropping it into a glass beaker containing 565 g of water initially at 20.0°C. The mass of the beaker is 50.0 g and the specific heat of the glass is 840 J/kg?K
What is the final equilibrium temperature of the system, assuming the cooling takes place very quickly, so that no energy is lost to the air? The specific heat of copper is 385 J/kg?K and that of water is 4190 J/kg?K.
26.4°C
You might also like to view...
A spherical flask, 4 m diameter with a 5 mm thick wall, is used to heat grape juice. During the heating process the outside surface of the flask is 100ºC and the inside surface is 80ºC. Estimate the thermal resistance of the flask, the heat transfer through the flask, if it is assumed that only the bottom half is heated, and the temperature distribution through the flask wall.
What will be an ideal response?
Simple Pendulum: A simple pendulum having a bob of mass M has a period T. If you double M but change nothing else, what would be the new period?
A. 2T
B. T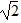
C. T
D. T/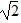
E. T/2
In stars, helium can sometimes be fused into carbon and heavier elements (in their final stages of life). Why didn't the same fusion processes produce carbon and heavier elements in the early universe?
A) By the time stable helium nuclei had formed, the temperature and density had already dropped too low for helium fusion to occur. B) Helium fusion occurred, but the carbon nuclei that were made were later destroyed by the intense radiation in the early universe. C) Temperatures in the early universe were never above the roughly 100 million Kelvin required for helium fusion. D) No one knows; this is one of the major mysteries in astronomy.
A crane lifts a 425 kg steel beam vertically upward a distance of 95m How much work does the crane do on the beam if the beam accelerates upward at 1.8 m/s2? Neglect frictional forces
A) 4.0 × 105 J B) 3.2 × 105 J C) 4.7 × 105 J D) 2.7 × 105J