Use the bond energies below to determine whether the following reaction is exothermic or endothermic:
H2+ CI2? 2 HCl
H-H (bond energy: 436 kJ/mol)
Cl-Cl (bond energy: 243 kJ/mol)
H-Cl (bond energy: 431 kJ/mol)
A) Exothermic with more than 50 kJ of energy released.
B) Endothermic with more than 50 kJ of energy absorbed.
C) Exothermic with less than 50 kJ of energy released.
D) Endothermic with less than 50 kJ of energy absorbed.
Answer: A
You might also like to view...
A force of 15.0 N is applied to a wire with an un-stretched length of 4.00 m and a diameter of 5.00 mm. If the wire changed length by 1.20 mm, then the strain in the wire is
a. 
b. 
c. 
d. 
e. 
Which of the following are true statements about the extrasolar planets found so far?
A) Extrasolar planets have a much wider range of densities than the planets in the solar system. B) Extrasolar planets fall neatly into the terrestrial and jovian categories. C) Extrasolar planets all have nearly circular orbits. D) All of the above are correct.
A machinist needs to punch a 5.0-cm-radius hole in a steel plate which is 0.45 cm thick. If the ultimate shear strength of steel is 2.50 × 10^8 Pa, what is the force required to accomplish this task?
a. 8.8E+5 N b. 3.5E+5 N c. 5.6E+6 N d. 1.1E+8 N
Equilibrium: An athlete holds a 7.5-kg shot put in his hand with his lower arm horizontal, as shown in the figure. His lower arm has a mass of 2.8 kg and its center of gravity (or center of mass) is 12 cm from the elbow-joint pivot. How much force must the extensor muscle (which is M in the figure) in the upper arm exert on the lower arm?
M in the figure) in the upper arm exert on the lower arm?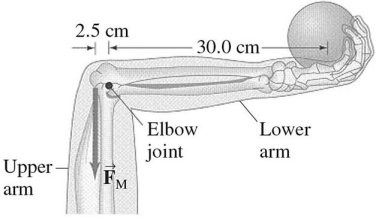
A. 100 N B. 500 N C. 1000 N D. 1500 N