First Law of Thermodynamics: The figure shows a pV diagram for a gas going through a cycle from A to B to C and back to A. From point A to point B, the gas absorbs 50 J of heat and finds its internal (thermal) energy has increased by 20 J. Going from B to C, the internal (thermal) energy decreases by 5.0 J.(a) How much work was done by the gas from A to B?(b) How much heat was absorbed by the gas from B to C?(c) How much work was done by the gas going from B to C?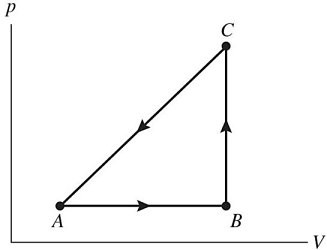
What will be an ideal response?
(a) 30 J (b) -5.0 (i.e. released 5.0 J) (c) 0 J
You might also like to view...
The ________ seismic waves can pass through both solid and liquid portions of the Earth's interior, and be detected on the other side of the globe
Fill in the blank(s) with correct word
The true masses of a spectroscopic binary cannot be found by astronomers
Indicate whether the statement is true or false
What is the mass density of argon gas at pressure 1.00 × 105 N/m2 and at temperature 300 K? The mean atomic mass of argon is 39.948 g/mol and the ideal gas constant is R = 8.314 J/mol ? K =
A) 1.40 kg/m3 B) 1.00 kg/m3 C) 1.20 kg/m3 D) 1.60 kg/m3 E) 1.80 kg/m3
A "double satellite" is launched into space. It consists of a satellite of mass m and another satellite of mass 5m. The connecting mechanism of these satellites is an explosive bolt and a compressed spring, both of negligible mass. The bolt is exploded and the satellites drift apart having been pushed by the spring. When the less massive satellite has moved a distance of 2.5 m from the center of
mass of the double satellite, how far away from it is the satellite of greater mass? a. 3.0 m c. 0.10 m b. 12 m d. 0.50 m