The 21 cm line of hydrogen is strongly absorbed by interstellar dust
Indicate whether the statement is true or false
FALSE
You might also like to view...
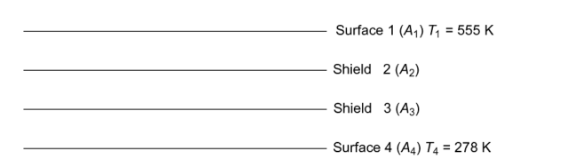
Determine the steady-state temperatures of two radiation shields placed in the evacuated space between two infinite planes at temperatures of 555 K and 278 K. The emissivity of all surfaces is 0.8.
GIVEN
-Two radiant shields placed in the evacuated space between two infinite planes
-Temperature of the planes
-T1 = 555 K
-T4 = 278 K
-Emissivity of all surface (?) = 0.8
FIND
-The steady state temperatures of the shields (T2, T3)
ASSUMPTIONS
• All surfaces are gray and diffuse
SKETCH
Metric System: An area of 1.00 × 102 cm2 is how many square meters?
A. 1.00 m2 B. 1.00 × 102 m2 C. 1.00 × 10-2 m2 D. 1.00 × 104 m2 E. 1.00 × 10-3 m2
Diatomic molecules such as 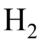 and
and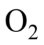 undergo simple harmonic motion with frequencies that obey Hooke’s Law with the effective mass being half the atomic mass. If the mass of an oxygen atom is 2.66 x
undergo simple harmonic motion with frequencies that obey Hooke’s Law with the effective mass being half the atomic mass. If the mass of an oxygen atom is 2.66 x 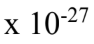 kg, and the observed frequency of oscillation is
kg, and the observed frequency of oscillation is 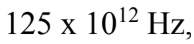 Hz, what is the effective spring constant associated with the bond between the atoms in an oxygen molecule
Hz, what is the effective spring constant associated with the bond between the atoms in an oxygen molecule 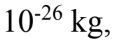
A. 1140 N/m B. 182 N/m C. 116 N/m D. 29 N/m E. 2290 N/m
Suppose you had molecular oxygen (O2 ) chilled enough so that it was in liquid form. Which of the following best describes the phase changes that would occur as you heated the liquid oxygen to high temperature?
A) It would evaporate into a gas, then the molecules would dissociate into individual oxygen atoms, then the atoms would become increasingly ionized as you continued to raise the temperature. B) The liquid molecules would quickly dissociate into a liquid of individual oxygen atoms. These atoms would then evaporate into a gas, and then become ionized to make a plasma. C) It would vaporize into a gas, then the molecules would lose electrons until no electrons were left, then the molecules would dissociate into individual oxygen nuclei. D) The cold temperature would first cause the oxygen to solidify. The solid would then sublimate into a gas, which would then become a plasma as the molecules lost their electrons, until finally it consisted of bonded pairs of oxygen nuclei stripped bare of any electrons.