The pH of an acidic solution is a measure of the concentration of the acid particles in the solution, with smaller values of the pH indicating higher acid concentration. In a laboratory experiment, the pH of a certain acid solution is changed by dissolving over-the-counter antacid tablets into the solution. In this experiment, the pH changes according to the equation
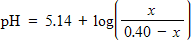
where x is the number of grams of antacid added to the solution. What is the pH of the solution after the addition of 0.05 grams of antacid tablet?
A. 5.99
B. 4.02
C. -0.85
D. 4.29
E. 5.14
Answer: D
You might also like to view...
State whether you think the difference between what occurred and what you would expect by chance is statistically significant.Of the people taking the medication, 38 out of 100 noticed improvement in their arthritis. Of the people taking the placebo, 35 out of 100 noticed improvement in their arthritis.
A. Not statistically significant B. Statistically significant
Estimate the magnitude of the error involved in using the sum of the first four terms to approximate the sum of the entire series.
A. 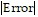 < 4.37 × 10-2
< 4.37 × 10-2
B. 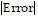 < 3.28 × 10-1
< 3.28 × 10-1
C. 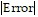 < 1.02 × 10-1
< 1.02 × 10-1
D.  < 6.55 × 10-2
< 6.55 × 10-2
Solve the equation.log6x = 2
A. x = 26
B. x = 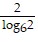
C. x = 62
D. x = 6?2
Multiply.(x - 3)(x - 2)
A. x2 + 6x - 5 B. x2 - 5x + 6 C. x2 - 5x - 6 D. x2 - 6x + 6