What is the mean free path for the molecules in an ideal gas when the pressure is 100 kPa and the temperature is 300 K given
that the collision cross-section for the molecules of that gas is 2.0 × 10-20 m2? Boltzmann's constant is k = 1.38 × 10-23 J/K.
A)
1.1 × 10-6 m
B)
2.1 × 10-6 m
C)
1.7 × 10-6 m
D)
5.3 × 10-6 m
E)
1.5 × 10-6 m
E
You might also like to view...
When a star has exhausted all of its hydrogen, it is said to have reached the zero-age main sequence (ZAMS)
Indicate whether the statement is true or false
H I clouds can be observed using _____
a. optical telescopes above the earth's atmosphere b. 21-cm radiation c. x-ray radiation d. infrared radiation e. ultraviolet radiation
The majority of dark matter is normal protons and neutrons
Indicate whether the statement is true or false
A planar cross section through two spherical waves emanating from the sources S1 and S2 in the plane is shown in the figure. S1 and S2 are in phase. The black circles are one and two wavelengths from their respective sources. The lighter circles are one half and one and a half wavelengths distant from their respective sources. If the waves shown
arriving at P2 both arrive with amplitude A, the resultant amplitude at point P2 is
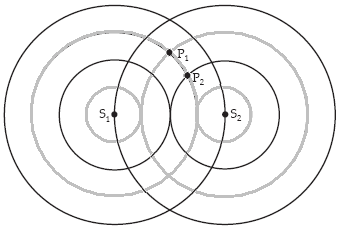
a.
0.
b.
.
c.
A.
d.