Balance the equation the following reaction representing the combustion of butane:
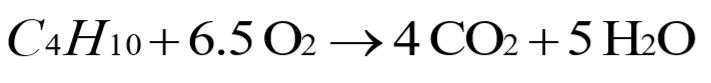
a. How many moles of oxygen are required to burn 1 mol of butane?
b. How many grams of oxygen are required to burn 1 kg of butane?
c. At standard temperature and pressure, what volume of oxygen would be required to burn 100 g of butane?
d. What volume of air at STP is required to burn 100 g of butane?
What will be an ideal response?
a.
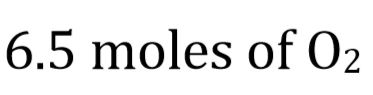
b.
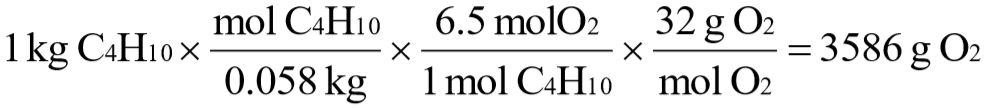
c.
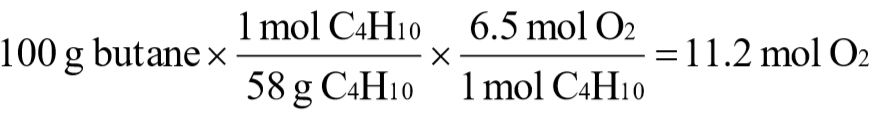
d.
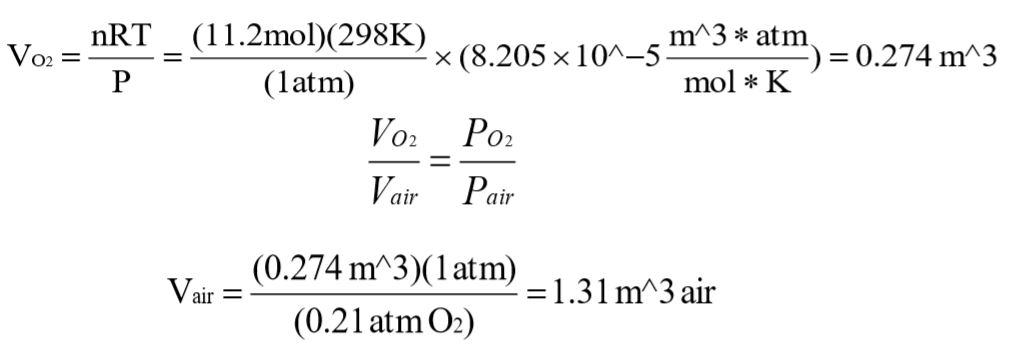
You might also like to view...
If valves are set loose, which of the following will result?
A. advanced valve timing B. hotter valve operating temperatures C. noisier valve operation D. retarded valve timing
Helena has 12,000 layers. The layers have a production life of 18 months with 82 percent production (82 percent of the days they will lay).
a. How many eggs are expected per hen in her production life? b. How many dozen eggs are expected per month? c. How many dozen eggs are expected in the production life of the 12,000 layers? Express your answer using scientific notation with the correct number of significant digits.
If a TEV system is overcharged to a 45° condenser split the symptoms will be ________.
a. higher head pressure and higher subcooling b. higher head pressure and normal subcooling c. higher head pressure and higher suction pressure d. higher head pressure and sweating on the compressor
Name the type of belt that would be used to convey a product to the end of an assembly line.
A. flat belt B. v-belt C. timing belt D. double V-belt